Abstract
Background
Artemisinin (ART) and its derivatives are important antimalaria agents and have received increased attention due to their broad biomedical effects, such as anticancer and anti-inflammation activities. Recently, ruthenium-derived complexes have attracted considerable attention as their anticancer potentials were observed in preclinical and clinical studies.
Methods
To explore an innovative approach in colorectal cancer (CRC) management, we synthesized ruthenium–dihydroartemisinin complex (D–Ru), a novel metal-based artemisinin derivative molecule, and investigated its anticancer, anti-inflammation, and adaptive immune regulatory properties.
Results
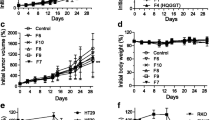
Compared with its parent compound, ART, D–Ru showed stronger antiproliferative effects on the human CRC cell lines HCT-116 and HT-29. The cancer cell inhibition of D–Ru comprised G1 cell cycle arrest via the downregulation of cyclin A and the induction of apoptosis. ART and D–Ru downregulated the expressions of pro-inflammatory cytokines IL-1β, IL-6, and IL-8. Although ART and D–Ru did not suppress Treg cell differentiation, they significantly inhibited Th1 and Th17 cell differentiation.
Conclusions
Our results demonstrated that D–Ru, a novel ruthenium complexation of ART, remarkably enhanced its parent compound’s anticancer action, while the anti-inflammatory potential was not compromised. The molecular mechanisms of action of D–Ru include inhibition of cancer cell growth via cell cycle arrest, induction of apoptosis, and anti-inflammation via regulation of adaptive immunity.








Similar content being viewed by others
Data availability
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Tang Y, Li X, Yuan Y et al (2022) Network pharmacology-based predictions of active components and pharmacological mechanisms of Artemisia annua L for the treatment of the novel Corona virus disease 2019 (COVID-19). BMC Complement Med Ther 22:56. https://doi.org/10.1186/s12906-022-03523-2
van der Kooy F, Sullivan SE (2013) The complexity of medicinal plants: the traditional Artemisia annua formulation, current status and future perspectives. J Ethnopharmacol 150:1–13. https://doi.org/10.1016/j.jep.2013.08.021
Feng X, Cao S, Qiu F, Zhang B (2020) Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol Ther 216:107650. https://doi.org/10.1016/j.pharmthera.2020.107650
Tu Y (2016) Artemisinin-a gift from traditional chinese medicine to the world (nobel lecture). Angew Chem Int Ed Engl 55:10210–10226. https://doi.org/10.1002/anie.201601967
Kong XJ, Liu KM, Zuo HL et al (2022) The changing global landscape in the development of artemisinin-based treatments: a clinical trial perspective. Am J Chin Med 50:733–748. https://doi.org/10.1142/S0192415X22500306
Ho WE, Peh HY, Chan TK, Wong WS (2014) Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 142:126–139. https://doi.org/10.1016/j.pharmthera.2013.12.001
Kim EJ, Kim GT, Kim BM et al (2017) Apoptosis-induced effects of extract from Artemisia annua Linne by modulating PTEN/p53/PDK1/Akt/ signal pathways through PTEN/p53-independent manner in HCT116 colon cancer cells. BMC Complement Altern Med 17:236. https://doi.org/10.1186/s12906-017-1702-7
Lin AJ, Klayman DL, Milhous WK (1987) Antimalarial activity of new water-soluble dihydroartemisinin derivatives. J Med Chem 30:2147–2150. https://doi.org/10.1021/jm00394a037
Alven S, Aderibigbe BA (2020) Nanoparticles formulations of artemisinin and derivatives as potential therapeutics for the treatment of cancer, leishmaniasis and malaria. Pharmaceutics. https://doi.org/10.3390/pharmaceutics12080748
Ndagi U, Mhlongo N, Soliman ME (2017) Metal complexes in cancer therapy - an update from drug design perspective. Drug Des Devel Ther 11:599–616. https://doi.org/10.2147/DDDT.S119488
Long DF, Repta AJ (1981) Cisplatin: chemistry, distribution and biotransformation. Biopharm Drug Dispos 2:1–16. https://doi.org/10.1002/bdd.2510020102
Huang W, Wang Y, He T et al (2022) Arteannuin B enhances the effectiveness of cisplatin in non-small cell lung cancer by regulating connexin 43 and MAPK pathway. Am J Chin Med 50:1963–1992. https://doi.org/10.1142/S0192415X22500847
Zhang JJ, Zhou YD, Liu YB et al (2021) Protective effect of 20(R)-ginsenoside Rg3 against cisplatin-induced renal toxicity via PI3K/AKT and NF-[Formula: see text]B signaling pathways based on the premise of ensuring anticancer effect. Am J Chin Med 49:1739–1756. https://doi.org/10.1142/S0192415X21500828
Sanna B, Debidda M, Pintus G et al (2002) The anti-metastatic agent imidazolium trans-imidazoledimethylsulfoxide-tetrachlororuthenate induces endothelial cell apoptosis by inhibiting the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway. Arch Biochem Biophys 403:209–218. https://doi.org/10.1016/s0003-9861(02)00218-7
Rademaker-Lakhai JM, van den Bongard D, Pluim D et al (2004) A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin Cancer Res 10:3717–3727. https://doi.org/10.1158/1078-0432.CCR-03-0746
Kostova I (2006) Ruthenium complexes as anticancer agents. Curr Med Chem 13:1085–1107. https://doi.org/10.2174/092986706776360941
Lentz F, Drescher A, Lindauer A et al (2009) Pharmacokinetics of a novel anticancer ruthenium complex (KP1019, FFC14A) in a phase I dose-escalation study. Anticancer Drugs 20:97–103. https://doi.org/10.1097/CAD.0b013e328322fbc5
McCarthy N (2013) Tumorigenesis: all together now. Nat Rev Cancer 13:148. https://doi.org/10.1038/nrc3469
Swierczynski M, Szymaszkiewicz A, Fichna J, Zielinska M (2020) New insights into molecular pathways in colorectal cancer: adiponectin, interleukin-6 and opioid signaling. Biochim Biophys Acta Rev Cancer 1875:188460. https://doi.org/10.1016/j.bbcan.2020.188460
Madka V, Rao CV (2013) Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr Cancer Drug Targets 13:542–557
Wang CZ, Yu C, Wen XD et al (2016) American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Cancer Prev Res (Phila) 9:803–811. https://doi.org/10.1158/1940-6207.CAPR-15-0372
Liang Y, Li C, Li L et al (2021) Artemisinin ruthenium metal complex, its preparation method and medical application in anti-tumor and anti-malaria. CN Patent CN2021–10184349 113150033
Yao H, Wan JY, Zeng J et al (2018) Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol Lett 15:8339–8348. https://doi.org/10.3892/ol.2018.8414
Wang CZ, Zhang CF, Luo Y et al (2020) Baicalein, an enteric microbial metabolite, suppresses gut inflammation and cancer progression in Apc(Min/+) mice. Clin Transl Oncol 22:1013–1022. https://doi.org/10.1007/s12094-019-02225-5
Liu H, Lu W, He H et al (2019) Inflammation-dependent overexpression of c-Myc enhances CRL4(DCAF4) E3 ligase activity and promotes ubiquitination of ST7 in colitis-associated cancer. J Pathol 248:464–475. https://doi.org/10.1002/path.5273
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/caac.21763
Siegel RL, Miller KD, Goding Sauer A et al (2020) Colorectal cancer statistics, 2020. CA Cancer J Clin 70:145–164. https://doi.org/10.3322/caac.21601
Schuurhuizen C, Braamse AMJ, Konings I et al (2017) Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? A systematic review. Ann Oncol 28:478–486. https://doi.org/10.1093/annonc/mdw617
Mehendale S, Aung H, Wang A et al (2005) American ginseng berry extract and ginsenoside Re attenuate cisplatin-induced kaolin intake in rats. Cancer Chemother Pharmacol 56:63–69. https://doi.org/10.1007/s00280-004-0956-1
Wang CZ, Fishbein A, Aung HH et al (2005) Polyphenol contents in grape-seed extracts correlate with antipica effects in cisplatin-treated rats. J Altern Complement Med 11:1059–1065. https://doi.org/10.1089/acm.2005.11.1059
Wang CZ, Luo X, Zhang B et al (2007) Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol 60:69–79. https://doi.org/10.1007/s00280-006-0350-2
Li XL, Wang CZ, Sun S et al (2009) American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep 22:943–952
Zeng L, Gupta P, Chen Y et al (2017) The development of anticancer ruthenium (ii) complexes: from single molecule compounds to nanomaterials. Chem Soc Rev 46:5771–5804. https://doi.org/10.1039/c7cs00195a
Wang Y, Bian L, Chakraborty T et al (2019) Construing the biochemical and molecular mechanism underlying the in vivo and in vitro chemotherapeutic efficacy of ruthenium-baicalein complex in colon cancer. Int J Biol Sci 15:1052–1071. https://doi.org/10.7150/ijbs.31143
El-Dallal M, Chen Y, Lin QY et al (2020) Meta-analysis of virtual-based chromoendoscopy compared with dye-spraying chromoendoscopy standard and high-definition white light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer Cancer. Inflamm Bowel Dis 26:1319–1329. https://doi.org/10.1093/ibd/izaa011
Ghanghas P, Jain S, Rana C, Sanyal SN (2016) Chemopreventive action of non-steroidal anti-inflammatory drugs on the inflammatory pathways in colon cancer. Biomed Pharmacother 78:239–247. https://doi.org/10.1016/j.biopha.2016.01.024
Garcia Rodriguez LA, Cea-Soriano L, Tacconelli S, Patrignani P (2013) Coxibs: pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res 191:67–93. https://doi.org/10.1007/978-3-642-30331-9_4
Song Z, Cheng K, Zhang L, Wang T (2017) Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol 69:184–190. https://doi.org/10.1016/j.jtherbio.2017.07.015
Lee AS, Hur HJ, Sung MJ (2020) The effect of artemisinin on inflammation-associated lymphangiogenesis in experimental acute colitis. Int J Mol Sci. https://doi.org/10.3390/ijms21218068
Yuan A, Chen JJ, Yao PL, Yang PC (2005) The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 10:853–865. https://doi.org/10.2741/1579
Cui G, Yuan A, Sun Z et al (2018) IL-1beta/IL-6 network in the tumor microenvironment of human colorectal cancer. Pathol Res Pract 214:986–992. https://doi.org/10.1016/j.prp.2018.05.011
Huang Y, Chen Z (2016) Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res 8:2490–2497
Oleinika K, Nibbs RJ, Graham GJ, Fraser AR (2013) Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol 171:36–45. https://doi.org/10.1111/j.1365-2249.2012.04657.x
Tauriello DVF, Palomo-Ponce S, Stork D et al (2018) TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–543. https://doi.org/10.1038/nature25492
Hou L, Yuki K (2022) CCR6 and CXCR6 identify the Th17 cells with cytotoxicity in experimental autoimmune encephalomyelitis. Front Immunol 13:819224. https://doi.org/10.3389/fimmu.2022.819224
Ueno A, Jeffery L, Kobayashi T et al (2018) Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun 87:38–49. https://doi.org/10.1016/j.jaut.2017.12.004
Acknowledgements
Not applicable.
Funding
This work was supported in part by the Tang Foundations, and the NIH/NIDDK grant 5P30DK042086.
Author information
Authors and Affiliations
Contributions
Project administration: CZW, CSY. Participated in research design: CHL, CFZ, QHZ, TLJ, LH. Conducted experiment: CZW, CW, GGL, YL, QM, AHW. Wrote an original draft preparation: CZW, ML, CSY. Performed data analysis: CW, YL. Edited and reviewed: CZW, CHL, TLJ, CSY.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Informed consent
Formal consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, CZ., Wan, C., Li, CH. et al. Ruthenium–dihydroartemisinin complex: a promising new compound for colon cancer prevention via G1 cell cycle arrest, apoptotic induction, and adaptive immune regulation. Cancer Chemother Pharmacol 93, 411–425 (2024). https://doi.org/10.1007/s00280-023-04623-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04623-7