Abstract
Purpose
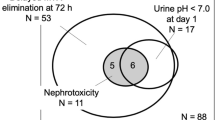
High dose methotrexate (HDMTX) acute kidney injury (AKI) results in prolonged hospitalization and treatment delays. Using a pharmacologically-based approach, HDMTX was administered with standard combination therapy to patients with osteosarcoma; nephrotoxicity was assessed.
Methods
Patients were randomized by cycle to 4 h or 12 h HDMTX (12 g/m2) infusions administered with hydration, alkalization and leucovorin rescue. Urinalysis, AKI biomarkers, and estimated glomerular filtration rate using serum creatinine or cystatin C (GFRCr or GFRcysC) were obtained. Serum and urine methotrexate concentrations [MTX] were measured.
Results
Patients (n = 12), median (range) age 12.4 (5.7–19.2) years were enrolled; 73 MTX infusions were analyzed. Median (95% Confidence Interval) serum and urine [MTX] were 1309 (1190, 1400) µM and 16.4 (14.7, 19.4) mM at the end of 4 h infusion and 557 (493, 586) µM and 11.1 (9.9, 21.1) mM at the end of 12 h infusion. Time to serum [MTX] < 0.1 µM was 83 (80.7, 90.7) h and 87 (82.8, 92.4) h for 4 and 12 h infusions. GFRCr was highly variable, increased after cisplatin, and exceeded 150 ml/min/1.73 m2. GFRcysC was less variable and decreased at the end of therapy. AKI biomarkers were elevated indicating acute tubular dysfunction, however, did not differ between 4 and 12 h infusions. Radiographic and histological response were similar for patients receiving 4 h or 12 h infusions; the median percent tumor necrosis was > 95%.
Conclusions
Reducing peak serum and urine MTX concentration by prolonging the infusion duration did not alter risk of acute kidney injury. GFRcysC was decreased at the end of therapy. Proteinuria and elevations in AKI biomarkers indicate that direct tubular damage contributes to HDMTX nephrotoxicity.
Clinical Trial
NCT01848457.



Similar content being viewed by others
References
Ritter J, Bielack SS (2010) Osteosarcoma. Ann Oncol 21(Suppl 7):vii320–vii325
Chou AJ et al (2009) Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer 115(22):5339–5348
Marina NM et al (2016) Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 17(10):1396–1408
Janeway KA, Grier HE (2010) Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol 11(7):670–678
Widemann BC et al (2004) High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer 100(10):2222–2232
Garneau AP, Riopel J, Isenring P (2015) Acute methotrexate-induced crystal nephropathy. N Engl J Med 373(27):2691–2693
Perazella MA (2015) The urine sediment as a biomarker of kidney disease. Am J Kidney Dis 66(5):748–755
Howard SC et al (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482
Ramsey LB et al (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23(1):52–61
Ferrari S et al (2009) Sex- and age-related chemotherapy toxicity in patients with non-metastatic osteosarcoma. J Chemother 21(2):205–210
Hempel L et al (2003) Influence of high-dose methotrexate therapy (HD-MTX) on glomerular and tubular kidney function. Med Pediatr Oncol 40(6):348–354
Jacobs SA et al (1976) 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest 57(2):534–538
Drost SA et al (2017) Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy 37(6):684–691
Treon SP, Chabner BA (1996) Concepts in use of high-dose methotrexate therapy. Clin Chem 42(8 Pt 2):1322–1329
Graf N et al (1994) Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 12(7):1443–1451
Ferrari S et al (1993) Serum methotrexate (MTX) concentrations and prognosis in patients with osteosarcoma of the extremities treated with a multidrug neoadjuvant regimen. J Chemother 5(2):135–141
Bacci G et al (2006) No correlation between methotrexate serum level and histologic response in the pre-operative treatment of extremity osteosarcoma. Anticancer Drugs 17(4):411–415
Zelcer S et al (2005) Methotrexate levels and outcome in osteosarcoma. Pediatr Blood Cancer 44(7):638–642
Crews KR et al (2004) High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 100(8):1724–1733
Krailo M et al (1987) A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group. Med Pediatr Oncol 15(2):69–77
Stevens LA et al (2006) Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483
Schwartz GJ et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20(3):629–637
He L et al (2019) The value of serum cystatin C in early evaluation of renal insufficiency in patients undergoing chemotherapy: a systematic review and meta-analysis. Cancer Chemother Pharmacol 83(3):561–571
Du Y et al (2011) Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol 26(2):267–274
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet 394(10212):1949–1964
Fox E et al (2018) Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin-related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist 23(7):762-e79
Turci R et al (2000) Determination of methotrexate in human urine at trace levels by solid phase extraction and high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 14(3):173–9
Barbieri A et al (2006) Simultaneous determination of low levels of methotrexate and cyclophosphamide in human urine by micro liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 20(12):1889–93
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106(3):522–6
Pedrosa DC, Meneses NFGC, Wirtzbiki G, Moraes C, Martins A, Liborio A (2015) Urinary KIM-1 in children undergoing antineoplstic treatment: a prospective cohort study. Pediatr Nephrol 30:2207–2213
Parikh CR et al (2013) Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8(7):1079–88
Acknowledgements
This work was funded by Gateway for Cancer Research Foundation and Alex's Lemonade Stand Center of Excellence.
Funding
This clinical trial was funded by the Gateway for Cancer Research and Alex's Lemonade Stand Center of Excellence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fox, E., Busch, C., DeBernardo, A. et al. A pharmacologically-based approach to high dose methotrexate administration to investigate nephrotoxicity and acute kidney injury biomarkers in children and adolescents with newly diagnosed osteosarcoma. Cancer Chemother Pharmacol 87, 807–815 (2021). https://doi.org/10.1007/s00280-021-04248-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04248-8