Abstract
Purpose
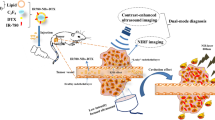
Molecule-targeted ultrasound imaging has attracted extensive attention for precise diagnosis and targeted therapy of tumors. The aim of this research is to prepare novel biomimetic dual-mode nanoscale ultrasound contrast agents (UCAs), which can not only evade the immune clearance of reticuloendothelial system, but also have the potential ability of precise detection and photothermal ablation of tumors.
Methods
In this study, for the first time, the novel biomimetic UCAs were prepared by encapsulating liquid perfluorohexanes with red blood cell membranes carrying IR-780 iodide and named IR780-RBCM@NDs. The characteristics of that were verified through the particle size analyzer, scanning electron microscopy, transmission electron microscopy and laser scanning confocal microscopy. The stability of IR780-RBCM@NDs at 37 °C was explored. The abilities of immune escape, dual-mode imaging and photothermal effect for IR780-RBCM@NDs were verified via in vitro experiments.
Results
The novel prepared nanodroplets have good characteristics such as mean diameter, zeta potential, and relatively stability. Importantly, the integrin-associated protein expressed on the surface of RBCMs was detected on IR780-RBCM@NDs. Then, compared with control groups, IR780-RBCM@NDs performed excellent immune escape function away from macrophages in vitro. Furthermore, the IR-780 iodide was observed on the new nanodroplets and that was able to perform the dual-mode imaging with near-infrared fluorescence imaging and contrast-enhanced ultrasound imaging after the phase change. Finally, the effective photothermal ablation ability of IR780-RBCM@NDs was verified in tumor cells.
Conclusion
The newly prepared biomimetic IR780-RBCM@NDs provided novel ideas for evading immune clearance, performing precise diagnosis and photothermal ablation of tumor cells.






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Bertier G, Carrot-Zhang J, Ragoussis V, Joly Y (2016) Integrating precision cancer medicine into healthcare—policy, practice, and research challenges. Genome Med 8:108. https://doi.org/10.1186/s13073-016-0362-4
Abou-Elkacem L, Bachawal SV, Willmann JK (2015) Ultrasound molecular imaging: Moving toward clinical translation. Eur J Radiol 84:1685–1693. https://doi.org/10.1016/j.ejrad.2015.03.016
Rix A, Lederle W, Theek B, Lammers T, Moonen C, Schmitz G, Kiessling F (2018) Advanced ultrasound technologies for diagnosis and therapy. J Nucl Med 59:740–746. https://doi.org/10.2967/jnumed.117.200030
Li M, Pei J, Ma Z, Fu J, Chen F, Du S (2020) Docetaxel-loaded ultrasmall nanostructured lipid carriers for cancer therapy: in vitro and in vivo evaluation. Cancer Chemoth Pharm 85:731–739. https://doi.org/10.1007/s00280-020-04048-6
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. P Natl Acad Sci USA 95:4607–4612. https://doi.org/10.1073/pnas.95.8.4607
Eroğlu İ, İbrahim M (2019) Liposome-ligand conjugates: a review on the current state of art. J Drug Target 13:1–20. https://doi.org/10.1080/1061186X.2019.1648479
Zhang L, Yi H, Song J, Huang J, Yang K, Tan B, Wang D, Yang N, Wang Z, Li X (2019) Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl Mater Inter 11:9355–9366. https://doi.org/10.1021/acsami.8b21968
Upadhyay S, Sharma N, Gupta KB, Dhiman M (2018) Role of immune system in tumor progression and carcinogenesis. J Cell Biochem 119:5028–5042. https://doi.org/10.1002/jcb.26663
Vijayan V, Uthaman S, Park IK (2018) Cell membrane coated nanoparticles: an emerging biomimetic nanoplatform for targeted bioimaging and therapy. Adv Exp Med Biol 1064:45–59. https://doi.org/10.1007/978-981-13-0445-3_3
Li R, He Y, Zhang S, Qin J, Wang J (2018) Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B 8:14–22. https://doi.org/10.1016/j.apsb.2017.11.009
Chen Z, Wu S, Chen P, Chen Y, Mou C (2019) Critical features for mesoporous silica nanoparticles encapsulated into erythrocytes. ACS Appl Mater Inter 11:4790–4798. https://doi.org/10.1021/acsami.8b18434
Hu CJ, Fang RH, Luk BT, Chen KNH, Carpenter C, Gao W, Zhang K, Zhang L (2013) ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5:2664. https://doi.org/10.1039/c3nr00015j
Rao L, Bu L, Xu J, Cai B, Yu G, Yu X, He Z, Huang Q, Li A, Guo S, Zhang W, Liu W, Sun Z, Wang H, Wang T, Zhao X (2015) Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small 11:6225–6236. https://doi.org/10.1002/smll.201502388
Yang H, Cai W, Lv W, Zhao P, Shen Y, Zhang L, Ma B, Yuan L, Duan Y, Yao K (2019) A new strategy for accurate targeted diagnosis and treatment of cutaneous malignant melanoma: dual-mode phase-change lipid nanodroplets as ultrasound contrast agents. Int J Nanomedicine 14:7079–7093. https://doi.org/10.2147/IJN.S207419
Zhu H, Qin D, Wu Y, Jing B, Liu J, Hazlewood D, Zhang H, Feng Y, Yang X, Wan M, Wu D (2018) Laser-activated bioprobes with high photothermal conversion efficiency for sensitive photoacoustic/ultrasound imaging and photothermal sensing. ACS Appl Mater Inter 10:29251–29259. https://doi.org/10.1021/acsami.8b08190
Alves CG, Lima-Sousa R, de Melo-DiOgo D, Louro RO, Correia IJ (2018) IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int J Pharm 542:164–175. https://doi.org/10.1016/j.ijpharm.2018.03.020
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Fraguas-Sánchez AI, Martín-Sabroso C, Fernández-Carballido A, Torres-Suárez AI (2019) Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemoth Pharm 84:689–706. https://doi.org/10.1007/s00280-019-03910-6
Yang C, Li Y, Du M, Chen Z (2019) Recent advances in ultrasound-triggered therapy. J Drug Target 27:33–50. https://doi.org/10.1080/1061186X.2018.1464012
Song J, Zhang L, Zhang N, Yi H, Huang J, Zhong Y, Hao L, Yang K, Wang D, Wang Z, Yang Z (2019) NIR-responsive nanoplatform for pre/intraoperative image-guided carcinoma surgery and photothermal ablation of residual tumor tissue. Nanomedicine 20:102020. https://doi.org/10.1016/j.nano.2019.102020
Yang H, Cai W, Xu L, Lv X, Qiao Y, Li P, Wu H, Yang Y, Zhang L, Duan Y (2015) Nanobubble–affibody: novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials 37:279–288. https://doi.org/10.1016/j.biomaterials.2014.10.013
de Leon A, Perera R, Nittayacharn P, Cooley M, Jung O, Exner AA (2018) Ultrasound contrast agents and delivery systems in cancer detection and therapy. Adv Cancer Res 139:57–84. https://doi.org/10.1016/bs.acr.2018.04.002
Strohm E, Rui M, Gorelikov I, Matsuura N, Kolios M (2011) Vaporization of perfluorocarbon droplets using optical irradiation. Biomed Opt Express 2:1432–1442. https://doi.org/10.1364/BOE.2.001432
Zhao H, Wu M, Zhu L, Tian Y, Wu M, Li Y, Deng L, Jiang W, Shen W, Wang Z, Mei Z, Li P, Ran H, Zhou Z, Ren J (2018) Cell-penetrating peptide-modified targeted drug-loaded phase-transformation lipid nanoparticles combined with low-intensity focused ultrasound for precision theranostics against hepatocellular carcinoma. Theranostics 8:1892–1910. https://doi.org/10.7150/thno.22386
Ma Y, Huang J, Song S, Chen H, Zhang Z (2016) Cancer-targeted nanotheranostics: recent advances and perspectives. Small 12:4936–4954. https://doi.org/10.1002/smll.201600635
Methachan B, Thanapprapasr K (2017) Polymer-based materials in cancer treatment: from therapeutic carrier and ultrasound contrast agent to theranostic applications. Ultrasound Med Biol 43:69–82. https://doi.org/10.1016/j.ultrasmedbio.2016.09.009
Jiang Q, Luo Z, Men Y, Yang P, Peng H, Guo R, Tian Y, Pang Z, Yang W (2017) Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials 143:29–45. https://doi.org/10.1016/j.biomaterials.2017.07.027
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S (2014) Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14:1891–1900. https://doi.org/10.1039/c4lc00136b
Jiang P, Narayanan V, Lagenaur CF (1999) Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 274:559–562. https://doi.org/10.1074/jbc.274.2.559
Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE (2013) Minimal "self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339:971–975. https://doi.org/10.1126/science.1229568
Hsieh CC, Kang ST, Lin YH, Ho YJ, Wang CH, Yeh CK, Chang CW (2015) Biomimetic acoustically-responsive vesicles for theranostic applications. Theranostics 11:1264–1274. https://doi.org/10.7150/thno.11848
Uthaman S, Mathew AP, Park HJ, Lee B, Kim H, Huh KM, Park I (2018) IR 780-loaded hyaluronic acid micelles for enhanced tumor-targeted photothermal therapy. Carbohydr Polym 181:1–9. https://doi.org/10.1016/j.carbpol.2017.10.033
Irajirad R, Ahmadi A, Najafabad BK, Abed Z, Sheervalilou R, Khoei S, Shiran MB, Ghaznavi H, Shakeri-Zadeh A (2019) Combined thermo-chemotherapy of cancer using 1 MHz ultrasound waves and a cisplatin-loaded sonosensitizing nanoplatform: an in vivo study. Cancer Chemoth Pharm 84:1315–1321. https://doi.org/10.1007/s00280-019-03961-9
Rhim H, Goldberg SN, Dodd GD, Solbiati L, Lim HK, Tonolini M, Cho OK (2001) Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics 21:S17–39. https://doi.org/10.1148/radiographics.21.suppl_1.g01oc11s17
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 81671706). The authors are grateful for the technical support and physical equipment provided by the Department of Molecular Biology of the Air Force Medical University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2020_4124_MOESM1_ESM.tif
Supplementary file1 S.Fig1. a The appearance and mean diameter of IR780-RBCM@NDs at 4 °C. Data were reported as the mean ± standard deviation (SD), n = 3. b The mean diameter of IR780-RBCM@NDs under optical microscope (40× objective lens) in 1, 3, 5, 7, and 14 days at 4 °C in vitro. The data represent the mean ± SD, n = 3 (TIF 8017 kb)
Rights and permissions
About this article
Cite this article
Liang, Y., Yang, H., Li, Q. et al. Novel biomimetic dual-mode nanodroplets as ultrasound contrast agents with potential ability of precise detection and photothermal ablation of tumors. Cancer Chemother Pharmacol 86, 405–418 (2020). https://doi.org/10.1007/s00280-020-04124-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04124-x