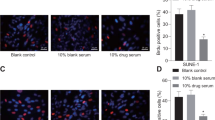
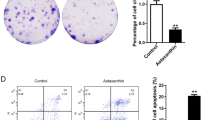
Abstract
Purpose
Brusatol, a natural quassinoid that is isolated from a traditional Chinese herbal medicine known as Bruceae Fructus, possesses biological activity in various types of human cancers, but its effects in nasopharyngeal carcinoma (NPC) have not been reported. This study aimed to explore the effect and molecular mechanism of brusatol in NPC in vivo and in vitro.
Methods
The antiproliferative effect of brusatol was assessed by MTT and colony formation assays. Apoptosis was determined by flow cytometry. The expression of mitochondrial apoptosis, cell cycle arrest, and Akt/mTOR pathway proteins were determined by western blot analysis. Further in vivo confirmation was performed in a nude mouse model.
Results
Brusatol showed antiproliferative activity against four human NPC cell lines (CNE-1, CNE-2, 5-8F, and 6-10B) in a dose-dependent manner. This antiproliferative effect was accompanied by mitochondrial apoptosis and cell cycle arrest through the modulation of several key molecular targets, such as Bcl-xl, Bcl-2, Bad, Bax, PARP, Caspase-9, Caspase-7, Caspase-3, Cdc25c, Cyclin B1, Cdc2 p34, and Cyclin D1. In addition, we found that brusatol inhibited the activation of Akt, mTOR, 4EBP1, and S6K, suggesting that the Akt/mTOR pathway is a key underlying mechanism by which brusatol inhibits growth and promotes apoptosis. Further in vivo nude mouse models proved that brusatol significantly inhibited the growth of CNE-1 xenografts with no significant toxicity.
Conclusions
These observations indicate that brusatol is a promising antitumor drug candidate or a supplement to current chemotherapeutic therapies to treat NPC.






Similar content being viewed by others
References
Carioli G, Negri E, Kawakita D et al (2017) Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int J Cancer 140(10):2256–2264. https://doi.org/10.1002/ijc.30660
Tang LL, Chen WQ, Xue WQ et al (2016) Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 374(1):22–30. https://doi.org/10.1016/j.canlet.2016.01.040
Miller KD, Nogueira L, Mariotto AB et al (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69(5):363–385. https://doi.org/10.3322/caac.21565
Tsang RK, Wei WI (2015) Salvage surgery for nasopharyngeal cancer. World J Otorhinolaryngol Head Neck Surg. 1(1):34–43. https://doi.org/10.1016/j.wjorl.2015.09.006
Chan JY, Tsang RK, Wei WI (2015) Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck 37(4):487–492. https://doi.org/10.1002/hed.23633
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet 394(10192):64–80. https://doi.org/10.1016/S0140-6736(19)30956-0
Fang W, Yang Y, Ma Y et al (2018) Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 19(10):1338–1350. https://doi.org/10.1016/S1470-2045(18)30495-9
Yin SY, Wei WC, Jian FY et al (2013) Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013:302426. https://doi.org/10.1155/2013/302426
Shan GY, Zhang S, Li GW et al (2011) Clinical evaluation of oral Fructus bruceae oil combined with radiotherapy for the treatment of esophageal cancer. Chin J Integr Med. 17(12):933–936. https://doi.org/10.1007/s11655-011-0953-2
Bai ZP, Deng XS (2010) Effect of oleum fructus brucease injection via bronchial arterial infusion in treating advanced lung cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 30(8):838–840. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=7&SID=5CvXb7A9fnrk1n7MO8s&page=1&doc=1
Wang B, Tian HQ, Liang GW (2009) Effect of ganji recipe combined with Fructus Bruceae oil emulsion intervention on quality of life in patients with advanced primary hepatic cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 29(3):257–260. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=13&SID=5CvXb7A9fnrk1n7MO8s&page=1&doc=1
Tian HQ, Yu SY, Wang B (2007) Effect of Fructus Bruceae oil emulsion on cellular immune function and quality of life in patients with non-small cell lung cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 27(2):157–159. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=19&SID=5CvXb7A9fnrk1n7MO8s&page=1&doc=1
Ye R, Dai N, He Q et al (2018) Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother 105:962–973. https://doi.org/10.1016/j.biopha.2018.06.065
Oh ET, Kim CW, Kim HG et al (2017) Brusatol-mediated inhibition of c-Myc increases HIF-1α degradation and causes cell death in colorectal cancer under hypoxia. Theranostics. 7(14):3415–3431. https://doi.org/10.7150/thno.20861
Evans JP, Winiarski BK, Sutton PA et al (2018) The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget. 9(43):27104–27116. https://doi.org/10.18632/oncotarget.25497
Lu Z, Lai ZQ, Leung AWN et al (2017) Exploring brusatol as a new anti-pancreatic cancer adjuvant: biological evaluation and mechanistic studies. Oncotarget. 8(49):84974–84985. https://doi.org/10.18632/oncotarget.17761
Xiang Y, Ye W, Huang C et al (2018) Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxid Med Cell Longev. 2018:2360427. https://doi.org/10.1155/2018/2360427
Lee JH, Rangappa S, Mohan CD et al (2019) Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules. https://doi.org/10.3390/biom9100550
Liu Y, Lu Y, Celiku O et al (2019) Targeting IDH1-Mutated Malignancies with NRF2 Blockade. J Natl Cancer Inst 111(10):1033–1041. https://doi.org/10.1093/jnci/djy230
Liu X, Xu H, Zhang Y et al (2019) Brusatol inhibits amyloid-β-induced neurotoxicity in U-251 cells via regulating the Nrf2/HO-1 pathway. J Cell Biochem 120(6):10556–10563. https://doi.org/10.1002/jcb.28341
Cai SJ, Liu Y, Han S et al (2019) Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 9:45. https://doi.org/10.1186/s13578-019-0309-8
Ren D, Villeneuve NF, Jiang T et al (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A. 108(4):1433–1438. https://doi.org/10.1073/pnas.1014275108
Olayanju A, Copple IM, Bryan HK et al (2015) Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic Biol Med. 78:202–212. https://doi.org/10.1016/j.freeradbiomed.2014.11.003
Chan SY, Choy KW, Tsao SW et al (2008) Authentication of nasopharyngeal carcinoma tumor lines. Int J Cancer. 122(9):2169–2171. https://doi.org/10.1002/ijc.23374
Zhang Z, Lee JC, Lin L et al (2012) Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 44(8):852–860. https://doi.org/10.1038/ng.2330
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Lu H, Peng L, Yuan X et al (2009) Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev 35(4):345–353. https://doi.org/10.1016/j.ctrv.2009.01.002
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17(2):93–115. https://doi.org/10.1038/nrc.2016.138
Curtin NJ (2012) DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 12(12):801–817. https://doi.org/10.1038/nrc3399
Sherr CJ (1996) Cancer cell cycles. Science 274(5293):1672–1677. https://doi.org/10.1126/science.274.5293.1672
Gordon EM, Ravicz JR, Liu S et al (2018) Cell cycle checkpoint control: the cyclin G1/Mdm2/p53 axis emerges as a strategic target for broad-spectrum cancer gene therapy—a review of molecular mechanisms for oncologists. Mol Clin Oncol. 9(2):115–134. https://doi.org/10.3892/mco.2018.1657
Pan Z, Zhang X, Yu P et al (2019) Cinobufagin induces cell cycle arrest at the G2/M phase and promotes apoptosis in malignant melanoma cells. Front Oncol. 9:853. https://doi.org/10.3389/fonc.2019.00853
Henriques AC, Ribeiro D, Pedrosa J et al (2019) Mitosis inhibitors in anticancer therapy: when blocking the exit becomes a solution. Cancer Lett 440–441:64–81. https://doi.org/10.1016/j.canlet.2018.10.005
Bock FJ, Tait SWG (2019) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. https://doi.org/10.1038/s41580-019-0173-8
Teng QX, Ashar YV, Gupta P et al (2019) Revisiting mTOR inhibitors as anticancer agents. Drug Discov Today. 24(10):2086–2095. https://doi.org/10.1016/j.drudis.2019.05.030
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 169(2):361–371. https://doi.org/10.1016/j.cell.2017.03.035
Roux PP, Topisirovic I (2018) Signaling pathways involved in the regulation of mRNA translation. Mol Cell Biol. https://doi.org/10.1128/MCB.00070-18
Xiang Y, Ye W, Huang C et al (2017) Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem Biophys Res Commun 487(4):820–826. https://doi.org/10.1016/j.bbrc.2017.04.133
Funding
This research was supported by the National College Students’ Innovation Entrepreneurship Training Plan Program of China (No. 201910570006 to S. G.), the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds) (No. pdjh2019b0403 to S. G.), the Guangzhou Medical University College Students Science Technology Innovation Project (No. 2018A019 to S. G.), the National Natural Science Foundation of China (No. 81672276 to Z. Z.), the Guangzhou Science and Technology Program (No. 201803010038 to Z. Z.), the National Natural Science Foundation of China (No. 21907017 to B. L.), the Guangdong Natural Science Foundation of China (No. 2018A030310186 to B. L.), the Science and Technology Planning Project of Guangdong Province of China (No. A2018377 to B. L.), and the Guangzhou Health and Family Planning Commission of Guangdong Province of China (No. 20181A011068 to B. L.).
Author information
Authors and Affiliations
Contributions
SG designed the study, performed the experiments, analyzed the data and drafted the manuscript. JZ, CW, ZL, RC, DP, HZ and BL participated in a part of the experiments. ZZ conceived the idea and decided on the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, S., Zhang, J., Wei, C. et al. Anticancer effects of brusatol in nasopharyngeal carcinoma through suppression of the Akt/mTOR signaling pathway. Cancer Chemother Pharmacol 85, 1097–1108 (2020). https://doi.org/10.1007/s00280-020-04083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04083-3