Abstract
Purpose
We aimed to investigate the association between the polymorphism and expression patterns of multiple drug resistance genes (MDR) in breast cancer (BC).
Materials and methods
The MDR gene expression levels were measured in tumor tissues of 106 breast cancer patients using quantitative real-time PCR. Affymetrix CytoScan™ HD Array chips were used to assess genotypes. Pairwise correlation analysis for ABCB1, ABCC1, ABCC2 and ABCG2 gene expression levels was carried out to reveal co-expression clusters. Associations between SNPs of MDR genes and their preoperative expression levels were assessed using analysis of covariance adjusting for covariates.
Results
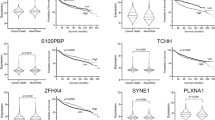
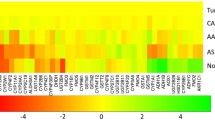
The SNPs associated with the expression of the ABCB1, ABCC1, ABCC2 and ABCG2 genes before NAC were detected. In addition, 21 SNPs associated with the expression of four ABC-transporter genes and involved in the expression regulation were identified. Validation in an independent sample confirmed the association between the MDR cluster genes and 11 SNPs.
Conclusions
Four MDR genes: ABCB1, ABCC1, ABCC2 and ABCG2 were shown to form the functional expression cluster in breast tumor. Further studies are required to discover precise mechanisms of the cluster regulation, thereby providing new approaches and targets to combat the development of the MDR phenotype during chemotherapy.

Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19(5):1508–1516
Fletcher JI, Haber M, Henderson MJ, Norris MD (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10(2):147–156
Szakacs G, Varadi A, Özvegy-Laczka C, Sarkadi B (2008) The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov Today 13(9):379–393
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234
Litviakov NV, Cherdyntseva NV, Tsyganov MM, Denisov EV, Garbukov EY, Merzliakova MK, Volkomorov VV, Vtorushin SV, Zavyalova MV, Slonimskaya EM (2013) Changing the expression vector of multidrug resistance genes is related to neoadjuvant chemotherapy response. Cancer Chemother Pharmacol 71(1):153–163
Litviakov NV (2013) Gradient phenomenon of multidrug resistance gene expression in breast cancer during neoadjuvant chemotherapy is related to disease progression. Sib J Oncol 4:58
Orsetti B, Courjal F, Cuny M, Rodriguez C, Theillet C (1999) 17q21-q25 aberrations in breast cancer: combined allelotyping and CGH analysis reveals 5 regions of allelic imbalance among which two correspond to DNA amplification. Oncogene 18(46):6262–6270
Ha G, Roth A, Khattra J, Ho J, Yap D, Prentice LM, Melnyk N, McPherson A, Bashashati A, Laks E (2014) TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res 24(11):1881–1893
Okudela K, Tateishi Y, Umeda S, Mitsui H, Suzuki T, Saito Y, Woo T, Tajiri M, Masuda M, Miyagi Y (2014) Allelic imbalance in the miR-31 host gene locus in lung cancer-its potential role in carcinogenesis. PLoS One 9(6):e100581
Fleming JL, Dworkin AM, Allain DC, Fernandez S, Wei L, Peters SB, Iwenofu OH, Ridd K, Bastian BC, Toland AE (2014) Allele-specific imbalance mapping identifies HDAC9 as a candidate gene for cutaneous squamous cell carcinoma. Int J Cancer 134(1):244–248
Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, Kalva S, Potter J, Tran TV, Chen J (2014) Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 16(6):475
Shen J, Medico L, Zhao H (2011) Allelic imbalance in BRCA1 and BRCA2 gene expression and familial ovarian cancer. Cancer Epidemiol Biomark Prev 20(1):50–56
Amiri-Kordestani L, Basseville A, Kurdziel K, Fojo AT, Bates SE (2012) Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updates 15(1):50–61
Nakanishi T, Ross DD (2012) Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 31(2):73
Saito M, Okamoto A, Kohno T, Takakura S, Shinozaki H, Isonishi S, Yasuhara T, Yoshimura T, Ohtake Y, Ochiai K (2000) Allelic imbalance and mutations of the PTEN gene in ovarian cancer. Int J Cancer 85(2):160–165
Natarajan K, Xie Y, Baer MR, Ross DD (2012) Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol 83(8):1084–1103
Tang SC, Lankheet NA, Poller B, Wagenaar E, Beijnen JH, Schinkel AH (2012) P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) restrict brain accumulation of the active sunitinib metabolite N-desethyl sunitinib. J Pharmacol Exp Ther 341(1):164–173
Vattathil S, Scheet P (2013) Haplotype-based profiling of subtle allelic imbalance with SNP arrays. Genome Res 23(1):152–158
Schwartz GF, Hortobagyi GN (2004) Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Breast J 10(4):273–294
Hayward J, Carbone P, Heuson J-C, Kumaoka S, Segaloff A (1965) Rubens R (1977) Assessment of response to therapy in advanced breast cancer: a project of the programme on clinical oncology of the international union against cancer, Geneva, Switzerland. Eur J Cancer 13(1):89–94
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29(9):e45
Litviakov NV, Cherdyntseva NV, Tsyganov MM, Slonimskaya EM, Ibragimova MK, Kazantseva PV, Kzhyshkowska J, Choinzonov EL (2016) Deletions of multidrug resistance gene loci in breast cancer leads to the down-regulation of its expression and predict tumor response to neoadjuvant chemotherapy. Oncotarget 7(7):7829
Kanzaki A, Toi M, Nakayama K, Bando H, Mutoh M, Uchida T, Fukumoto M, Takebayashi Y (2001) Expression of multidrug resistance-related transporters in human breast carcinoma. Jpn J Cancer Res 92(4):452–458
Lacave R, Coulet F, Ricci S, Touboul E, Flahault A, Rateau JG, Cesari D, Lefranc JP, Bernaudin JF (1998) Comparative evaluation by semiquantitative reverse transcriptase polymerase chain reaction of MDR1, MRP and GSTp gene expression in breast carcinomas. Br J Cancer 77(5):694–702
Larkin A, O’Driscoll L, Kennedy S, Purcell R, Moran E, Crown J, Parkinson M, Clynes M (2004) Investigation of MRP-1 protein and MDR-1 P-glycoprotein expression in invasive breast cancer: a prognostic study. Int J Cancer 112(2):286–294. doi:10.1002/ijc.20369
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, Stoter G, Nooter K (2003) RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res 9(2):827–836
Kim B, Fatayer H, Hanby AM, Horgan K, Perry SL, Valleley EM, Verghese ET, Williams BJ, Thorne JL, Hughes TA (2013) Neoadjuvant chemotherapy induces expression levels of breast cancer resistance protein that predict disease-free survival in breast cancer. PLoS One 8(5):e62766
Moureau-Zabotto L, Ricci S, Lefranc JP, Coulet F, Genestie C, Antoine M, Uzan S, Lotz JP, Touboul E, Lacave R (2006) Prognostic impact of multidrug resistance gene expression on the management of breast cancer in the context of adjuvant therapy based on a series of 171 patients. Br J Cancer 94(4):473–480. doi:10.1038/sj.bjc.6602958
Ma H, Zhou H, Song X, Shi S, Zhang J, Jia L (2014) Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene 34:726–740. doi:10.1038/onc.2014.7
Tanida S, Mizoshita T, Ozeki K, Tsukamoto H, Kamiya T, Kataoka H, Sakamuro D, Joh T (2012) Mechanisms of cisplatin-induced apoptosis and of cisplatin sensitivity: potential of BIN1 to act as a potent predictor of cisplatin sensitivity in gastric cancer treatment. Int J Surg Oncol 2012:862879-1–862879-8. doi:10.1155/2012/862879
Park DC, Yeo SG, Shin EY, Mok SC, Kim DH (2006) Clusterin confers paclitaxel resistance in cervical cancer. Gynecol Oncol 103(3):996–1000
Hicks C, Kumar R, Pannuti A, Miele L (2012) Integrative analysis of response to tamoxifen treatment in ER-positive breast cancer using GWAS information and transcription profiling. Breast Cancer 6:47
Iusuf D, Teunissen SF, Wagenaar E, Rosing H, Beijnen JH, Schinkel AH (2011) P-glycoprotein (ABCB1) transports the primary active tamoxifen metabolites endoxifen and 4-hydroxytamoxifen and restricts their brain penetration. J Pharmacol Exp Ther 337(3):710–717
Teft WA, Mansell SE, Kim RB (2011) Endoxifen, the active metabolite of tamoxifen, is a substrate of the efflux transporter P-glycoprotein (multidrug resistance 1). Drug Metab Dispos 39(3):558–562
Goler-Baron V, Sladkevich I, Assaraf YG (2012) Inhibition of the PI3K-Akt signaling pathway disrupts ABCG2-rich extracellular vesicles and overcomes multidrug resistance in breast cancer cells. Biochem Pharmacol 83(10):1340–1348
Zheng T, Wang J, Chen X, Liu L (2010) Role of microRNA in anticancer drug resistance. Int J Cancer 126(1):2–10
Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S (2010) Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol 79(6):817–824
Sharma G, Mirza S, Parshad R, Srivastava A, Datta Gupta S, Pandya P, Ralhan R (2010) CpG hypomethylation of MDR1 gene in tumor and serum of invasive ductal breast carcinoma patients. Clin Biochem 43(4):373–379
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The study is funded by the Russian Science Foundation No. 17-15-01203 “Metastatic breast tumor clones”.
Informed consent
Informed consent was obtained from all individual participants included in the study. The experiments comply with the current laws of the country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsyganov, M.M., Freidin, M.B., Ibragimova, M.K. et al. Genetic variability in the regulation of the expression cluster of MDR genes in patients with breast cancer. Cancer Chemother Pharmacol 80, 251–260 (2017). https://doi.org/10.1007/s00280-017-3354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3354-1