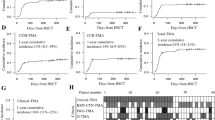
Abstract
Hematopoietic stem cell transplantation-associated thrombotic microangiopathy (TA-TMA) is a fatal post-transplant complication. It has a high mortality rate and worse prognosis, but treatment strategies remain controversial. We screened 6 out of 3453 studies on the treatment of TA-TMA. These investigations compared 5 treatment strategies with a network meta-analysis approach. The final outcome was the proportion of patients who responded to these therapies. There were significant differences in response rates for each treatment. Achieving analysis through direct and indirect evidence in the rank probabilities shows that rTM (recombinant human soluble thrombomodulin) is most likely to be rank 1 (64.98%), Eculizumab intervention rank 2 (48.66%), ISM (immunosuppression manipulation) rank 3 (32.24%), TPE (therapeutic plasma exchange) intervention rank 4 (69.56%), and supportive care intervention rank 5 (70.20%). Eculizumab and ISM have significantly higher efficacy than supportive care (odds ratio (OR): 18.04, 18.21 respectively); and TPE having lower efficacy than all other TA-TMA therapies exception to supportive care. In our study, rTM and Eculizumab may be the best choice when treating TA-TMA.





Similar content being viewed by others
References
Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J et al (2014) Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 124(4):645–653. https://doi.org/10.1182/blood-2014-03-564997
Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM et al (2015) A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev 29(3):191–204. https://doi.org/10.1016/j.blre.2014.11.001
Renaghan AD, Jaimes EA, Malyszko J, Perazella MA, Sprangers B, Rosner MH (2020) Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin J Am Soc Nephrol CJASN 15(2):289–297. https://doi.org/10.2215/cjn.08580719
Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ et al (2010) Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 90(8):918–926. https://doi.org/10.1097/TP.0b013e3181f24e8d
Qi J, Wang J, Chen J, Su J, Tang Y, Wu X et al (2017) Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann Hematol 96(11):1849–1855. https://doi.org/10.1007/s00277-017-3092-9
Jan AS, Hosing C, Aung F, Yeh J (2019) Approaching treatment of transplant-associated thrombotic microangiopathy from two directions with eculizumab and transitioning from tacrolimus to sirolimus. Transfusion 59(11):3519–3524. https://doi.org/10.1111/trf.15534
Kennedy GA, Kearey N, Bleakley S, Butler J, Mudie K, Durrant S (2010) Transplantation-associated thrombotic microangiopathy: effect of concomitant GVHD on efficacy of therapeutic plasma exchange. Bone Marrow Transplant 45(4):699–704. https://doi.org/10.1038/bmt.2009.233
Bohl SR, Harsdorf S, Schoensteiner S, Schwarzwaelder P, Scholl K, Wais V et al (2016) Eculizumab therapy of adult TA-TMA: a high response rate is associated with a high infection-related mortality. Blood 128(22)
Ikezoe T, Yang J, Nishioka C, Honda G, Furihata M, Yokoyama A (2012) Thrombomodulin protects endothelial cells from a calcineurin inhibitor-induced cytotoxicity by upregulation of extracellular signal-regulated kinase/myeloid leukemia cell-1 signaling. Arterioscler Thromb Vasc Biol 32(9):2259–2270. https://doi.org/10.1161/atvbaha.112.251157
Maruyama I (1999) Recombinant thrombomodulin and activated protein C in the treatment of disseminated intravascular coagulation. Thromb Haemost 82(2):718–721
Corti P, Uderzo C, Tagliabue A, Della Volpe A, Annaloro C, Tagliaferri E et al (2002) Defibrotide as a promising treatment for thrombotic thrombocytopenic purpura in patients undergoing bone marrow transplantation. Bone Marrow Transplant 29(6):542–543. https://doi.org/10.1038/sj.bmt.1703414
Salanti G, Higgins JP, Ades AE, Ioannidis JP (2008) Evaluation of networks of randomized trials. Stat Methods Med Res 17(3):279–301. https://doi.org/10.1177/0962280207080643
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
GA. W, B. S, D. OC, Peterson. J, Welch. V, Losos. M, et al (2021) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta–analysis. Available at:http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (Accessed December 12, 2021).
Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS (1998) Interpreting treatment effects in randomised trials. BMJ (Clin Res ed) 316(7132):690–693. https://doi.org/10.1136/bmj.316.7132.690
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Shim SR, Kim SJ, Lee J, Rücker G (2019) Network meta-analysis: application and practice using R software. Epidemiol Health 41:e2019013. https://doi.org/10.4178/epih.e2019013
Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. (2007) A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess (Winchester England). 11(14):1–160, iii-iv. https://doi.org/10.3310/hta11140
Epperla N, Hemauer K, Hamadani M, Friedman KD, Kreuziger LB (2017) Impact of treatment and outcomes for patients with posttransplant drug-associated thrombotic microangiopathy. Transfusion 57(11):2775–2781. https://doi.org/10.1111/trf.14263
Fujiwara H, Maeda Y, Sando Y, Nakamura M, Tani K, Ishikawa T et al (2016) Treatment of thrombotic microangiopathy after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Transfusion 56(4):886–892. https://doi.org/10.1111/trf.13437
Rudoni J, Jan A, Hosing C, Aung F, Yeh J (2018) Eculizumab for transplant-associated thrombotic microangiopathy in adult allogeneic stem cell transplant recipients. Eur J Haematol 101(3):389–398. https://doi.org/10.1111/ejh.13127
Narimatsu H, Kami M, Hara S, Matsumura T, Miyakoshi S, Kusumi E et al (2005) Intestinal thrombotic microangiopathy following reduced-intensity umbilical cord blood transplantation. Bone Marrow Transplant 36(6):517–523. https://doi.org/10.1038/sj.bmt.1705099
Imus PH, Tsai HL, DeZern AE, Jerde K, Swinnen LJ, Bolaños-Meade J et al (2020) Thrombotic microangiopathy after post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 26(12):2306–2310. https://doi.org/10.1016/j.bbmt.2020.09.018
Sakai M, Ikezoe T, Bandobashi K, Togitani K, Yokoyama A (2010) Successful treatment of transplantation-associated thrombotic microangiopathy with recombinant human soluble thrombomodulin. Bone Marrow Transplant 45(4):803–805. https://doi.org/10.1038/bmt.2009.242
Choi CM, Schmaier Ah Fau - Snell MR, Snell Mr Fau - Lazarus HM, Lazarus HM. (n.d) Thrombotic microangiopathy in haematopoietic stem cell transplantation: diagnosis and treatment. (0012–6667 (Print)).
Howard Ma Fau - Williams LA, Williams La Fau - Terrell DR, Terrell Dr Fau - Duvall D, Duvall D Fau - Vesely SK, Vesely Sk Fau - George JN, George JN. (n.d) Complications of plasma exchange in patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. (0041–1132 (Print)).
Mulay S, Kreuter JD, Bryant SC, Elliott MA, Hogan WJ, Winters JL et al (2015) Outcomes of plasma exchange in patients with transplant-associated thrombotic microangiopathy based on time of presentation since transplant. J Clin Apheresis 30(3):147–153. https://doi.org/10.1002/jca.21352
Jodele S, Laskin BL, Goebel J, Khoury JC, Pinkard SL, Carey PM et al (2013) Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion 53(3):661–667. https://doi.org/10.1111/j.1537-2995.2012.03776.x
Jodele S (2018) Complement in pathophysiology and treatment of transplant-associated thrombotic microangiopathies. Semin Hematol 55(3):159–166. https://doi.org/10.1053/j.seminhematol.2018.04.003
Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J et al (2016) Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 22(2):307–315. https://doi.org/10.1016/j.bbmt.2015.10.002
Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink-Cross A, Myers KC, et al (n.d) Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. (1528–0020 (Electronic)).
Lebranchu Y, Snanoudj R, Toupance O, Weestel PF, de Ligny BH, Buchler M et al (2012) Five-year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: The Spiesser Study. Am J Transplant 12(7):1801–1810. https://doi.org/10.1111/j.1600-6143.2012.04036.x
Olejarz W, Bryk D, Zapolska-Downar D, Malecki M, Stachurska A, Sitkiewicz D (2014) Mycophenolic acid attenuates the tumour necrosis factor-alpha-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-kappaB and ROS pathways. Eur J Clin Invest 44(1):54–64. https://doi.org/10.1111/eci.12191
Freguin-Bouilland C, Godin M, Bellien J, Richard V, Remy-Jouet I, Dautreaux B et al (2011) Protective effect of mycophenolate mofetil on endothelial function in an aortic allograft model. Transplantation 91(1):35–41. https://doi.org/10.1097/TP.0b013e3181fe12d6
Suzuki K, Hayashi T, Nishioka J, Kosaka Y, Zushi M, Honda G et al (1989) A domain composed of epidermal growth factor-like structures of human thrombomodulin is essential for thrombin binding and for protein C activation. J Biol Chem 264(9):4872–4876
Pan B, Wang X, Kojima S, Nishioka C, Yokoyama A, Honda G et al (2017) The fifth epidermal growth factor like region of thrombomodulin alleviates LPS-induced sepsis through interacting with GPR15. Thromb Haemost 117(3):570–579. https://doi.org/10.1160/th16-10-0762
Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M et al (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115(5):1267–1274. https://doi.org/10.1172/jci22782
Van de Wouwer M, Plaisance S Fau - De Vriese A, De Vriese A Fau - Waelkens E, Waelkens E Fau - Collen D, Collen D Fau - Persson J, Persson J Fau - Daha MR, et al. (n.d) The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. (1538–7933 (Print)).
Saito H, Maruyama I Fau - Shimazaki S, Shimazaki S Fau - Yamamoto Y, Yamamoto Y Fau - Aikawa N, Aikawa N Fau - Ohno R, Ohno R Fau - Hirayama A, et al. (n.d) Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. (1538–7933 (Print)).
Funding
This work was supported by National Natural Science Foundation of China (81873432 and 82070143), grants from the Jiangsu Province of China (BE2021645), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Jingyi Yang and Xiaoyan Xu designed and performed research studies, analyzed the data, and wrote the manuscript. Shiyu Han and Jiaqian Qi performed research studies and analyzed data. Xueqian Li and Rui Zhang contributed to the data analysis. Tingting Pan was responsible for the correction of chart and data. Yue Han contributed to the research design, data analysis, writing of the manuscript, and supervision of the study.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingyi Yang, Xiaoyan Xu, and Shiyu Han are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Xu, X., Han, S. et al. Comparison of multiple treatments in the management of transplant-related thrombotic microangiopathy: a network meta-analysis. Ann Hematol 102, 31–39 (2023). https://doi.org/10.1007/s00277-022-05069-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-05069-2