Abstract
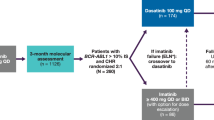
Tyrosine kinase inhibitors (TKIs), the backbone of treatment for chronic phase chronic myeloid leukemia patients (CP-CML), have changed the long-term outcome of the disease. Nonetheless, over 20% of patients fail front-line therapy due to intolerance or resistance. A head-to-head comparison of dasatinib and nilotinib as second-line treatment outside of sponsored clinical trials has not been reported. We retrospectively analyzed 131 CP-CML patients who, after front-line imatinib failure, switched to a second-line therapy with nilotinib (59, 45%) or dasatinib (72, 55%). Median duration of second-line treatment was 33 months (range 2–100). The reason for switching therapy was resistance in 83.2% and intolerance in 16.8% of patients. The overall survival of the entire cohort at 7 years was 78.9%, while it was 72% and 85.6% for patients treated with dasatinib and nilotinib, respectively (p=0.287). With regard to efficacy after 12 months of treatment, 108 patients were evaluable for molecular response: 47% achieved a major molecular response and 18.2% a deep molecular response with dasatinib, compared to 38% and 16.2% with nilotinib (p=ns). We observed 35% of grade 3–4 adverse events, more frequently in the dasatinib group (47%) compared to the nilotinib group (22%), without affecting molecular responses. Our study suggests that, in the real-life setting, dasatinib and nilotinib used as second-line treatment in CP-CML are equally effective, with high molecular response rates and an acceptable tolerability.





Similar content being viewed by others
References
Melo JV, Hughes TP, Apperley JF (2003) Chronic myeloid leukemia. Hematol Am Soc Hematol Educ Program 2003:132–152
Rowley JD (1973) Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243(5405):290–293
Bower H, Bjorkholm M, Dickman PW et al (2016) Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 34(24):2851–2857
O’Brien SG, Guilhot F, Larson RA et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348(11):994–1004
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ, IRIS Investigators (2017) Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 376(10):917–927
Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG (2006) Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354(24):2542–2551
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL (2006) Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354(24):2531–2541
Rogers G, Hoyle M, Thompson Coon J, Moxham T, Liu Z, Pitt M, Stein K (2012) Dasatinib and nilotinib for imatinib-resistant or -intolerant chronic myeloid leukaemia: a systematic review and economic evaluation. Health Technol Assess 16(22):1–410
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, Clark RE, Cortes JE, Deininger MW, Guilhot F, Hjorth-Hansen H, Hughes TP, Janssen JJWM, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Mayer J, Nicolini F, Niederwieser D, Pane F, Radich JP, Rea D, Richter J, Rosti G, Rousselot P, Saglio G, Saußele S, Soverini S, Steegmann JL, Turkina A, Zaritskey A, Hehlmann R (2020) European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 34(4):966–984
Matsumura I, Ohtake S, Atsuta Y, et al. Nilotinib vs dasatinib in achieving MR4.5 for newly diagnosed chronic myeloid leukemia: results of the prospective randomized phase 3 study, JALS CML212. ASH 2020: abstract 45
de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, Szydlo R, Olavarria E, Kaeda J, Goldman JM, Marin D (2008) Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol 26(20):3358–3363
Lucas CM, Wang L, Austin GM, Knight K, Watmough SJ, Shwe KH, Dasgupta R, Butt NM, Galvani D, Hoyle CF, Seale JRC, Clark RE (2008) A population study of imatinib in chronic myeloid leukaemia demonstrates lower efficacy than in clinical trials. Leukemia 22(10):1963–1966
Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, Hochhaus A, Radich JP, Saglio G, Hughes TP, Martinelli G, Kim DW, Shou Y, Gallagher NJ, Blakesley R, Baccarani M, Cortes J, le Coutre PD (2011) Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 117(4):1141–1145
Shah NP, Rousselot P, Schiffer C, Rea D, Cortes JE, Milone J, Mohamed H, Healey D, Kantarjian H, Hochhaus A, Saglio G (2016) Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol 91(9):869–874
Cojbasic I, Mačukanović-Golubović L, Vučić M et al (2017) Analyses of treatment outcome according to age in patients with chronic myeloid leukemia receiving front-line imatinib therapy. Clin Lymphoma Myeloma Leuk 17(10):696–702
Breccia M, Tiribelli M, Alimena G (2012) Tyrosine kinase inhibitors for elderly chronic myeloid leukemia patients: a systematic review of efficacy and safety data. Crit Rev Oncol Hematol 84(1):93–100
Giles FJ, Rea D, Rosti G, Cross NCP, Steegmann JL, Griskevicius L, le Coutre P, Coriu D, Petrov L, Ossenkoppele GJ, Mahon FX, Saussele S, Hellmann A, Koskenvesa P, Brümmendorf TH, Gastl G, Castagnetti F, Vincenzi B, Haenig J, Hochhaus A (2017) Impact of age on efficacy and toxicity of nilotinib in patients with chronic myeloid leukemia in chronic phase: ENEST1st subanalysis. J Cancer Res Clin Oncol 143(8):1585–1596
Molica M, Canichella M, Alunni Fegatelli D, Colafigli G, Massaro F, Latagliata R, Foà R, Breccia M (2017) The Eutos long-term survival score accurately predicts the risk of death in chronic myeloid leukaemia patients treated outside of clinical trials. Am J Hematol 92(12):E661–E664
Griffin JD, Guerin A, Chen L, Macalalad AR, Luo J, Ionescu-Ittu R, Wu EQ (2013) Comparing nilotinib with dasatinib as second-line therapies in patients with chronic myelogenous leukemia resistant or intolerant to imatinib -- a retrospective chart review analysis. Curr Med Res Opin 29(6):623–631
Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, Hochhaus A, Radich JP, Saglio G, Hughes TP, Martinelli G, Kim DW, Novick S, Gillis K, Fan X, Cortes J, Baccarani M, Kantarjian HM (2013) Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 27(1):107–112
Tiribelli M, Bonifacio M, Binotto G, Iurlo A, Cibien F, Maino E, Guella A, Festini G, Minotto C, de Biasi E, de Marchi F, Scaffidi L, Frison L, Bucelli C, Medeot M, Calistri E, Sancetta R, Stulle M, Orofino N, Krampera M, Gherlinzoni F, Semenzato G, Pizzolo G, Ambrosetti A, Fanin R (2018) Excellent outcomes of 2G-TKI therapy after imatinib failure in chronic phase CML patients. Oncotarget 9(18):14219–14227
Garcia-Gutierrez V, Hernandez-Boluda JC (2019) Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol 9:603
Naqvi K, Jabbour E, Skinner J, Anderson K, Dellasala S, Yilmaz M, Ferrajoli A, Bose P, Thompson P, Alvarado Y, Jain N, Takahashi K, Burger J, Estrov Z, Borthakur G, Pemmaraju N, Paul S, Cortes J, Kantarjian HM (2020) Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 126(1):67–75
Efficace F, Stagno F, Iurlo A et al Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia 34(2):488–498
Author information
Authors and Affiliations
Contributions
GC, LR, GC, ADP, SP, IC, and RL collected data; DAF analyzed and produced statistical reports; DD performed the molecular analysis; GLN, MM, RF, and FE critically revised the paper; and ES and MB collected data and finalized and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the responsible institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Formal consent was obtained.
Conflict of interest
MB received honoraraia by Novartis, Pfizer, Incyte, BMS/Celgene, and AbbVie; FE reports personal fees from AbbVie, Bristol Myers Squibb, Orsenix, and Takeda; and grants (awarded to his institution) and personal fees from Amgen, outside the submitted work. MM received honoraria for advisory board outside of CML programs by Novartis, Roche, Gilead, Takeda, Janssen, and Celgene. All the other authors reported no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 343 kb)
Rights and permissions
About this article
Cite this article
Scalzulli, E., Caocci, G., Efficace, F. et al. Real-life comparison of nilotinib versus dasatinib as second-line therapy in chronic phase chronic myeloid leukemia patients. Ann Hematol 100, 1213–1219 (2021). https://doi.org/10.1007/s00277-021-04477-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04477-0