Abstract
Purpose
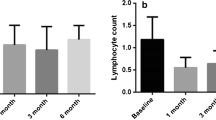
Neutrophil-to-lymphocyte ratio (NLR) recently demonstrated predictive value for hepatocellular carcinoma (HCC) recurrence after thermal ablation. Microwave ablation (MWA) has been shown to induce changes in the immune landscape after HCC treatment. This study aims at identifying predictors of local tumor progression (LTP) and post-treatment NLR kinetics after MWA.
Materials and Methods
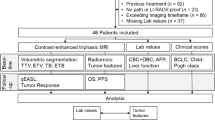
Data from 108 consecutive patients who underwent percutaneous MWA of 119 HCCs with a 2450 Hz/100 W generator in two institutions from October 2014 to September 2021 were retrospectively reviewed. Forty-five HCCs (42 patients) met inclusion criteria for analysis (technique efficacy, pre- and post-treatment NLR availability, follow-up > 6 months, absence of complications). NLR was analyzed prior to therapy and at 1-month follow-up; difference between the two time points was defined as ΔNLR1stFU.
Results
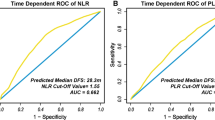
After a median follow-up of 25 months, LTP occurred in 18 HCCs (40%) and 18 patients (42.9%). Multivariate competing risk regression comprising ΔNLR1stFU > 0, cirrhosis etiology and subcapsular location showed that the only independent predictor of LTP was ΔNLR1stFU > 0, on both a per-patient (HR = 2.7, p = 0.049) and per-tumor (HR = 2.8, p = 0.047) analysis. ΔNLR1stFU > 0 occurred in 24/42 patients (57.1%). In this subgroup, higher rates of female patients (p = 0.026), higher mean baseline NLR (p < 0.0001) and lower mean energy/size (p = 0.006) were observed. Upon ROC curve analysis, energy/size < 1414 J/mm predicted ΔNLR1stFU > 0 with 76% sensitivity and 70% specificity (AUC = 0.74).
Conclusion
NLR increase after ablation was the only independent predictor of LTP, supporting the role of balance between systemic inflammation and immunity in recurrence after MWA. Ablation energy/tumor size predicted NLR increase, reinforcing the concept of immune ablation.
Level of Evidence
III.





Similar content being viewed by others
References
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25:74–85.
Xu XL, Di LX, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287:461–72.
Di Sandro S, Benuzzi L, Lauterio A, Botta F, De Carlis R, Najjar M, et al. Single hepatocellular carcinoma approached by curative-intent treatment: a propensity score analysis comparing radiofrequency ablation and liver resection. Eur J Surg Oncol. 2019;45(9):1691–9.
Kim JS, Kim W, So YH, Yu SJ, Kim BG. Topographical impact of hepatitis B-related hepatocellular carcinoma on local recurrence after radiofrequency ablation. J Clin Gastroenterol. 2014;48:66–72.
Lai ZC, Liang JY, Da CL, Wang Z, Ruan SM, Xie XY, et al. Do hepatocellular carcinomas located in subcapsular space or in proximity to vessels increase the rate of local tumor progression? A meta-analysis Life Sci. 2018;207:381–5.
Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280:300–12.
Kono M, Inoue T, Kudo M, Chishina H, Arizumi T, Takita M, et al. Radiofrequency ablation for hepatocellular carcinoma measuring 2 cm or smaller: results and risk factors for local recurrence. Dig Dis. 2014;32:670–7.
Chen Y, Yang Y, Xyuan Z, Qsheng F, Li X, Xin YJ, et al. Nomogram based on neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio to predict recurrence in patients with hepatocellular carcinoma after radiofrequency ablation. Cardiovasc Intervent Radiol. 2021;44:1551–60.
An C, Li WZ, Huang ZM, Yu XL, Han YZ, Liu FY, et al. Small single perivascular hepatocellular carcinoma: comparisons of radiofrequency ablation and microwave ablation by using propensity score analysis. Eur Radiol. 2021;31:4764–73.
Glassberg MB, Ghosh S, Clymer JW, Qadeer RA, Ferko NC, Sadeghirad B, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and metaanalysis. Onco Targets Ther. 2019;12:6407–38.
Nie Y, Yang D, Oppenheim JJ. Alarmins and antitumor immunity. Clin Ther. 2016;38:1042–53.
Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120–34.
Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–46.
Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–57.
Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289:816–30.
Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria-A 10-year update. Radiology. 2014;273:241–60.
Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–8.
Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–75.
Puijk RS, Ahmed M, Goldberg SN, Meijerink MR. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;301:533–40.
Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014. https://doi.org/10.1093/jnci/dju124.
Hung HC, Lee JC, Cheng CH, Wu TH, Wang YC, Lee CF, et al. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 2017;24:559–69.
Johnson PJ, Dhanaraj S, Berhane S, Bonnett L, Ma YT. The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: a prospective controlled study. Br J Cancer. 2021;125:714–6.
Masucci MT, Minopoli M, Carriero MV. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis Prognosis and Therapy. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.01146.
Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal. 2019;17:1–11.
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–55.
Li XF, Chen DP, Ouyang FZ, Chen MM, Wu Y, Kuang DM, et al. Increased autophagy sustains the survival and pro-tumourigenic effects of neutrophils in human hepatocellular carcinoma. J Hepatol. 2015;62:131–9.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48.
Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS ONE. 2013;8(3):58184.
Zhang H, Hou X, Cai H, Zhuang X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim Invasive Ther Allied Technol. 2017;26:207–11.
Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:893–907.
Nijkamp MW, Van Der Bilt JDW, De Bruijn MT, Molenaar IQ, Voest EE, Van Diest PJ, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814–23.
Rozenblum N, Zeira E, Bulvik B, Gourevitch S, Yotvat H, Galun E, et al. Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology. 2015;276:416–25.
Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery. 2006;139:73–81.
Rozenblum N, Zeira E, Scaiewicz V, Bulvik B, Gourevitch S, Yotvat H, et al. Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology. 2015;276:426–32.
Markezana A, Ahmed M, Kumar G, Zorde-Khvalevsky E, Rozenblum N, Galun E, et al. Moderate hyperthermic heating encountered during thermal ablation increases tumor cell activity. Int J Hyperth. 2020;37:119–29.
Mehta A, Oklu R, Sheth RA. Thermal ablative therapies and immune checkpoint modulation: can locoregional approaches effect a systemic response? Gastroenterol Res Pract. 2016;2016:1–11.
Ahmad F, Gravante G, Bhardwaj N, Strickland A, Basit R, West K, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg. 2010;200:500–6.
Velez E, Goldberg SN, Kumar G, Wang Y, Gourevitch S, Sosna J, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281:782–92.
Alonzo M, Bos A, Bennett S, Ferral H. The Emprint™ Ablation system with thermosphere™ technology: one of the newer next-generation microwave ablation technologies. Semin Intervent Radiol. 2015;32:335–8.
Jilma B, Eichler HG, Breiteneder H, Wolzt M, Aringer M, Graninger W, et al. Effects of 17 beta-estradiol on circulating adhesion molecules. J Clin Endocrinol Metab. 1994;79:1619–24.
Bouman A, Jan Heineman M, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23.
Gardini AC, Marisi G, Canale M, Foschi FG, Donati G, Ercolani G, et al. Radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of overall survival and recurrence-free survival. Onco Targets Ther. 2018;11:6555–67.
Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–9.
Liu WR, Tian MX, Tang Z, Fang Y, Zhou YF, Song SS, et al. Nine-factor-based immunohistochemistry classifier predicts recurrence for early-stage hepatocellular carcinoma after curative resection. Br J Cancer. 2020;123:92–100.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Consent to Publish
Patients also signed informed consent regarding publishing their data and diagnostic images.
Ethical Standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Della Corte, A., Sallemi, C., Ratti, F. et al. Retrospective Evaluation and Significance of Neutrophil-to-Lymphocyte Ratio Prior to and 1 month Following Microwave Ablation of Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 46, 49–59 (2023). https://doi.org/10.1007/s00270-022-03288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03288-8