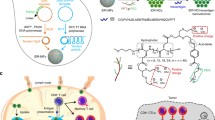
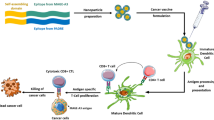
Abstract
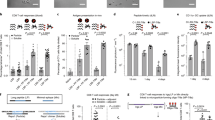
Neoantigen vaccines constitute an emerging and promising cancer immunotherapy. However, not all neoantigens have anti-tumor activity, as poor CD4+ epitope recognition can lead to the lack of greatly limit the persistence of the CD8+ T cell response. Therefore, we designed a self-assembled nanoplatform hereinafter referred to as DNA-coupled nitrated T helper cell epitope nanoparticle (DCNP) based on DNA origami containing a nitrated CD4 + T cell epitope, which can facilitate the effective activation of neoantigen-specific CD8+ T cells. Moreover, we embedded the cytidine-phosphate-guanosine oligonucleotide (CpG ODN) motif sequence in the DNA skeleton to function as a built-in adjuvant to activate Toll-like receptor 9. DCNP can markedly improve adjuvant and neoantigen co-delivery to lymphoid organs and promote neoantigen presentation on dendritic cells. Moreover, DCNP induced robust, and long-lived neoantigen-specific CD8+ T cell responses that significantly delayed tumor growth. Further, these effects were largely dependent on the nitrated T cell epitope. Collectively, our findings indicate that DCNP is a promising platform that could improve the development of personalized therapeutic neoantigen vaccines for cancer immunotherapy.






Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- BMDCs:
-
Bone marrow-derived dendritic cells
- CpG:
-
Cytosine–phosphate–guanine
- CpG ODN:
-
Cytidine–phosphate–guanosine oligonucleotide
- CTLs:
-
Cytotoxic T lymphocytes
- DCs:
-
Dendritic cells
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ELISA:
-
Enzyme-linked immunosorbent assay
- ELISPOT:
-
IFN-γ enzyme-linked immunospot
- FBS:
-
Fetal bovine serum
- LNs:
-
Lymph nodes
- MHC-I:
-
Major histocompatibility complex class I
- NitraTh:
-
Nitrated T helper cell epitope
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate-buffered saline
- Poly-ICLC:
-
Polyinosinic–polycytidylic acid
- SLPs:
-
Synthetic long peptides
- TEM:
-
Transmission electron microscopy
- TILs:
-
Tumor-infiltrating lymphocytes
- TLR9:
-
Toll-like receptor 9
References
Vormehr M, Türeci Ö, Sahin U (2019) Harnessing tumor mutations for truly individualized cancer vaccines. Annu Rev Med 70:395–407. https://doi.org/10.1146/annurev-med-042617-101816
Li L, Goedegebuure SP, Gillanders WE (2017) Preclinical and clinical development of neoantigen vaccines. Annal Oncol. https://doi.org/10.1093/annonc/mdx681
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348:69–74. https://doi.org/10.1126/science.aaa4971
Coulie PG, Van den Eynde BJ, van der Bruggen P et al (2014) Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14:135–146. https://doi.org/10.1038/nrc3670
Manoutcharian K, Guzman Valle J, Gevorkian G (2021) Neoantigen cancer vaccines: real opportunity or another illusion? Arch Immunol Ther Exp (Warsz) 69:12. https://doi.org/10.1007/s00005-021-00615-8
Wells DK, van Buuren MM, Dang KK et al (2020) Key Parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183(3):818–834. https://doi.org/10.1016/j.cell.2020.09.015
Blass E, Ott PA (2021) Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 18(4):215–229. https://doi.org/10.1038/s41571-020-00460-2
Carreno BM, Magrini V, Becker-Hapak M et al (2015) A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348:803–808. https://doi.org/10.1126/science.aaa3828
Keskin DB, Anandappa AJ, Sun J et al (2019) Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565:234–239. https://doi.org/10.1038/s41586-018-0792-9
Hu Z, Leet DE, Allesøe RL et al (2021) Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med 27:515–525. https://doi.org/10.1038/s41591-020-01206-4
Lynn GM, Sedlik C, Baharom F et al (2020) Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol 38:320–332. https://doi.org/10.1038/s41587-019-0390-x
Provine NM, Larocca RA, Aid M et al (2016) Immediate dysfunction of vaccine-elicited CD8+ T cells primed in the absence of CD4+ T cells. J Immunol 197:1809–1822. https://doi.org/10.4049/jimmunol.1600591
Laidlaw BJ, Craft JE, Kaech SM (2016) The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat Rev Immunol 16:102–111. https://doi.org/10.1038/nri.2015.10
Sahin U, Derhovanessian E, Miller M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222–226. https://doi.org/10.1038/nature23003
Lam H, McNeil LK, Starobinets H et al (2021) An empirical antigen selection method identifies neoantigens that either elicit broad antitumor t-cell responses or drive tumor growth. Cancer Discov 11:696–713. https://doi.org/10.1158/2159-8290.CD-20-0377
Zhou S, Zhong Q, Wang Y et al (2022) Chemically engineered mesoporous silica nanoparticles-based intelligent delivery systems for theranostic applications in multiple cancerous/non-cancerous diseases. Coord Chem Rev 452:214–309. https://doi.org/10.1016/j.ccr.2021.214309
Zamani P, Teymouri M, Nikpoor AR et al (2020) Nanoliposomal vaccine containing long multi-epitope peptide E75-AE36 pulsed PADRE-induced effective immune response in mice TUBO model of breast cancer. Eur J Cancer 129:80–96. https://doi.org/10.1016/j.ejca.2020.01.010
Moynihan KD, Opel CF, Szeto GL et al (2016) Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 22:1402–1410. https://doi.org/10.1038/nm.4200
Tian H, Kang Y, Song X et al (2020) PDL1-targeted vaccine exhibits potent antitumor activity by simultaneously blocking PD1/PDL1 pathway and activating PDL1-specific immune responses. Cancer Lett 476:170–182. https://doi.org/10.1016/j.canlet.2020.02.024
Tian H, He Y, Song X et al (2018) Nitrated T helper cell epitopes enhance the immunogenicity of HER2 vaccine and induce anti-tumor immunity. Cancer Lett 430:79–87. https://doi.org/10.1016/j.canlet.2018.05.021
Hor JL, Whitney PG, Zaid A et al (2015) Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43:554–565. https://doi.org/10.1016/j.immuni.2015.07.020
Bauer S, Kirschning CJ, Häcker H et al (2001) Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci 98:9237–9242. https://doi.org/10.1073/pnas.161293498
Lutz MB, Kukutsch N, Ogilvie ALJ et al (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92. https://doi.org/10.1016/S0022-1759(98)00204-X
Anthony DD, Lehmann PV (2003) T-cell epitope mapping using the ELISPOT approach. Methods 29:260–269. https://doi.org/10.1016/S1046-2023(02)00348-1
Li J, Pei H, Zhu B et al (2011) Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of Immunostimulatory CpG oligonucleotides. ACS Nano 5:8783–8789. https://doi.org/10.1021/nn202774x
Ballas ZK, Krieg AM, Warren T et al (2001) Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs1. J Immunol 167:4878–4886. https://doi.org/10.4049/jimmunol.167.9.4878
Reddy ST, Rehor A, Schmoekel HG et al (2006) In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release 112:26–34. https://doi.org/10.1016/j.jconrel.2006.01.006
Harms Jerome S, Khan M, Hall C et al (2018) Brucella peptide cross-reactive major histocompatibility complex class I presentation activates SIINFEKL-specific T cell receptor-expressing T cells. Infect Immun 86:e00281-e318. https://doi.org/10.1128/IAI.00281-18
Hung CF, Tsai YC, He L et al (2007) DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther 15:1211–1219. https://doi.org/10.1038/sj.mt.6300121
Fan C, Long R, You Y et al (2018) A novel PADRE-Kv1.3 vaccine effectively induces therapeutic antibodies and ameliorates experimental autoimmune encephalomyelitis in rats. Clin Immunol 193:98–109. https://doi.org/10.1016/j.clim.2018.02.012
Aqbi HF, Wallace M, Sappal S et al (2018) IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J Leukoc Biol 103:1219–1223. https://doi.org/10.1002/JLB.5MIR0917-351R
Deng S, Sun Z, Qiao J et al (2020) Targeting tumors with IL-21 reshapes the tumor microenvironment by proliferating PD-1intTim-3–CD8+ T cells. JCI Insight. 5(7):e132000. https://doi.org/10.1172/jci.insight.132000
Fischer DS, Ansari M, Wagner KI et al (2021) Single-cell RNA sequencing reveals ex vivo signatures of SARS-CoV-2-reactive T cells through ‘reverse phenotyping.’ Nat Commun 12:4515. https://doi.org/10.1038/s41467-021-24730-4
Azizi E, Carr AJ, Plitas G et al (2018) Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174(1293–1308):e36. https://doi.org/10.1016/j.cell.2018.05.060
Ranganathan YM, Shu C et al (2020) Single-cell transcriptome mapping identifies common and cell-type specific genes affected by acute delta9-tetrahydrocannabinol in humans. Sci Rep 10:3450. https://doi.org/10.1038/s41598-020-59827-1
Weng N-P, Liu K, Catalfamo M et al (2002) IL-15 is a growth factor and an activator of CD8 memory T cells. Ann N Y Acad Sci 975:46–56. https://doi.org/10.1111/j.1749-6632.2002.tb05940.x
Khanna KMT (2017) Understanding memory CD8+ T cells. Immunol Lett 185:32–39. https://doi.org/10.1016/j.imlet.2017.02.012
Reading JL, Gálvez-Cancino F, Swanton C et al (2018) The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol Rev 283:194–212. https://doi.org/10.1111/imr.12657
Snook AE, Magee MS, Schulz S et al (2014) Selective antigen-specific CD4+ T-cell, but not CD8+ T- or B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol 44:1956–1966. https://doi.org/10.1002/eji.201444539
Jiang L, Jiang T, Luo J et al (2019) Efficient acquisition of fully human antibody genes against self-proteins by sorting single B cells stimulated with vaccines based on nitrated T helper cell epitopes. J Immunol Res 2019:1–16. https://doi.org/10.1155/2019/7914326
Alexander J, Sidney J, Southwood S et al (1994) Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1:751–761. https://doi.org/10.1016/s1074-7613(94)80017-0
Morse MA, Gwin WR, Mitchell DA (2021) Vaccine therapies for cancer: then and now. Target Oncol 16:121–152. https://doi.org/10.1007/s11523-020-00788-w
Nasseri S, Kim A (2019) Selection of immunodominant epitopes during antigen processing is hierarchical. Mol Immunol 113:115–119. https://doi.org/10.1016/j.molimm.2018.08.011
Neek M, Kim TI, Wang S-W (2019) Protein-based nanoparticles in cancer vaccine development. Nanomedicine 15:164–174. https://doi.org/10.1016/j.nano.2018.09.004
Jiang SQ, Liu S et al (2018) A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol 36:258–264. https://doi.org/10.1038/nbt.4071
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 82073754, No. 81973222, No. 82273840), the Key R & D Program of Xinjiang Uygur Autonomous Region (2020B03003), and the “Double First-Class” University Project (No. CPU2018GF08).
Author information
Authors and Affiliations
Contributions
YK and WZ contributed equally to this study. YK and HT designed the project, conducted data analyses. HT, YK and WZ wrote the manuscript. WZ, QY, LG, JQ, FG, XW, YT, ZW, SS, HZ, and YZ were involved in the animal studies. LZ, XG, HT, and WY conceived the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that this study has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Y., Zhang, W., Yu, Q. et al. Self-assembled nanoparticles based on DNA origami and a nitrated T helper cell epitope as a platform for the development of personalized cancer vaccines. Cancer Immunol Immunother 72, 2741–2755 (2023). https://doi.org/10.1007/s00262-023-03446-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03446-y