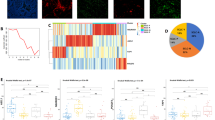
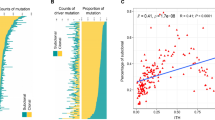
Abstract
Background
SCLC is an aggressive malignancy where immunotherapies show limited efficacy. We aimed to characterize the SCLC microenvironment according to the expression patterns of SCLC subtype markers and novel immune checkpoints to identify therapeutic vulnerabilities.
Methods
We included SCLC tissue samples from 219 surgically resected, limited-stage patients in this cross-sectional study. We performed immunohistochemistry for STING and MHCII, as well as for the novel subtype markers (ASCL1, NEUROD1, POU2F3, YAP1). Moreover, we assessed CD45 + , CD8 + and CD68 + immune cell infiltration.
Results
36% of SCLC tumors showed significant stromal or intraepithelial CD45 + immune cell infiltration. These patients exhibited significantly increased overall survival (OS) (vs. patients with immune-deserted tumors). High CD8 expression was associated with increased median OS. We found STING expression on cancer-associated fibroblasts in the stroma and on T-cells and macrophages in both tumorous and stromal compartments. STING expression positively correlated with immune cell infiltration. Increased STING-positivity in tumor nests was an independent favorable prognosticator for OS. ASCL1 was the most frequently expressed subtype-specific protein. Concomitant expression of three or four subtype-defining markers was seen in 13.8% of the included samples, whereas 24.1% of the cases were classified as quadruple negative tumors. YAP1 expression was associated with increased immune infiltrates. Tumor cell MHCII expression positively correlated with immune cell infiltration and with STING- and YAP1 expressions.
Conclusions
STING and MHCII are expressed in SCLC. The majority of immune-infiltrated SCLCs exhibit increased STING expression. Immune infiltration and STING expression are prognostic in limited-stage SCLC, making STING a potential therapeutic target.







Similar content being viewed by others
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
References
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 37(28):2518–2527. https://doi.org/10.1200/JCO.19.00934
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T (2019) KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 380(12):1116–1127. doi: https://doi.org/10.1056/NEJMoa1816714
Horn L, Mansfield AS, Szczȩsna A, Havel L, Krzakowski M, Hochmair MJ, Liu SV (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Williamson M (2019) Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet 394(10212):1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
Galon J, Bruni D (2020) Tumor immunology and tumor evolution: intertwined histories. Immunity 52(1):55–81. https://doi.org/10.1016/j.immuni.2019.12.018
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. https://doi.org/10.1126/science.1129139
Vesalainen S, Lipponen P, Talja M, Syrjänen K (1994) Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30A(12):1797–1803. https://doi.org/10.1016/0959-8049(94)e0159-2
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24(34):5373–5380. https://doi.org/10.1200/JCO.2006.05.9584
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77(7):1303–1310
Lin Z, Gu J, Cui X, Huang L, Li S, Feng J, Liu B, Zhou Y (2019) Deciphering microenvironment of NSCLC based on CD8+ TIL density and PD-1/PD-L1 expression. J Cancer 10(1):211–222. https://doi.org/10.7150/jca.26444
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61. https://doi.org/10.1016/j.immuni.2014.06.010. Erratum in: Immunity. 2014 Nov 20;41(5):866
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612. https://doi.org/10.1158/0008-5472.CAN-05-4005
Dora D, Rivard C, Yu H, Bunn P, Suda K, Ren S, Lueke Pickard S, Laszlo V, Harko T, Megyesfalvi Z, Moldvay J, Hirsch FR, Dome B, Lohinai Z (2020) Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint molecule distribution. Mol Oncol 14(9):1947–1965. https://doi.org/10.1002/1878-0261.12741
Kambayashi T, Laufer TM (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? nature reviews immunology. Nature Publishing Group
Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, Schreiber RD (2019) MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574(7780):696–701. https://doi.org/10.1038/s41586-019-1671-8
Dora D, Rivard C, Yu H, Pickard SL, Laszlo V, Harko T, Megyesfalvi Z, Dinya E, Gerdan C, Szegvari G, Hirsch FR, Dome B, Lohinai Z (2021) Characterization of tumor-associated macrophages and the immune microenvironment in limited-stage neuroendocrine-high and -low small cell lung cancer. Biology (Basel) 10(6):502. https://doi.org/10.3390/biology10060502
Barber GN (2015) STING: Infection, inflammation, and cancer nature reviews immunology. Nature Publishing Group
Corrales L, Gajewski TF (2015) Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer. Clin Cancer Res 21(21):4774–4779. https://doi.org/10.1158/1078-0432.CCR-15-1362
Rivera Vargas, T., Benoit-Lizon, I., & Apetoh, L. (2017) Rationale for stimulator of interferon genes targeted cancer immunotherapy European Journal of Cancer. Elsevier Ltd, Amsterdam
Kitajima S, Ivanova E, Guo S, Yoshida R, Campisi M, Sundararaman SK, Tange S, Mitsuishi Y, Thai TC, Masuda S, Piel BP, Sholl LM, Kirschmeier PT, Paweletz CP, Watanabe H, Yajima M, Barbie DA (2019) Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer. Cancer Discov. 9(1):34–45. https://doi.org/10.1158/2159-8290.CD-18-0689
Lohinai Z, Dora D, Caldwell C, Rivard CJ, Suda K, Yu H, Rivalland G, Ellison K, Rozeboom L, Dziadziuszko R, Mitchell P, John T, Millan IS, Ren S, Hirsch FR (2022) Loss of STING expression is prognostic in non-small cell lung cancer. J Surg Oncol 125(6):1042–1052. https://doi.org/10.1002/jso.26804
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D et al (2019) Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19:289–297
Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, Beras A, Spencer R, Lopardo J, Bodd F, Montecalvo J, Sauter JL, Chang JC, Buonocore DJ, Travis WD, Sen T, Poirier JT, Rudin CM, Rekhtman N (2020) SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 15(12):1823–1835. https://doi.org/10.1016/j.jtho.2020.09.009
Megyesfalvi Z, Barany N, Lantos A, Valko Z, Pipek O, Lang C, Schwendenwein A, Oberndorfer F, Paku S, Ferencz B, Dezso K, Fillinger J, Lohinai Z, Moldvay J, Galffy G, Szeitz B, Rezeli M, Rivard C, Hirsch FR, Brcic L, Popper H, Kern I, Kovacevic M, Skarda J, Mittak M, Marko-Varga G, Bogos K, Renyi-Vamos F, Hoda MA, Klikovits T, Hoetzenecker K, Schelch K, Laszlo V, Dome B (2022) Expression patterns and prognostic relevance of subtype-specific transcription factors in surgically resected small-cell lung cancer: an international multicenter study. J Pathol. https://doi.org/10.1002/path.5922
Schwendenwein A, Megyesfalvi Z, Barany N, Valko Z, Bugyik E, Lang C, Ferencz B, Paku S, Lantos A, Fillinger J, Rezeli M, Marko-Varga G, Bogos K, Galffy G, Renyi-Vamos F, Hoda MA, Klepetko W, Hoetzenecker K, Laszlo V, Dome B (2021) Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics 6(20):470–483. https://doi.org/10.1016/j.omto.2021.02.004
Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H (2000) Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127:3913–3921
Ikematsu Y, Tanaka K, Toyokawa G, Ijichi K, Ando N, Yoneshima Y, Iwama E, Inoue H, Tagawa T, Nakanishi Y, Okamoto I (2020) NEUROD1 is highly expressed in extensive-disease small cell lung cancer and promotes tumor cell migration. Lung Cancer 146:97–104. https://doi.org/10.1016/j.lungcan.2020.05.012
Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO (2012) The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA 109:E2441–E2450
Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, Hirota J (2017) Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One 12(12):e0189340. https://doi.org/10.1371/journal.pone.0189340
Huang YH, Klingbeil O, He XY, Wu XS, Arun G, Lu B, Somerville TDD, Milazzo JP, Wilkinson JE, Demerdash OE, Spector DL, Egeblad M, Shi J, Vakoc CR (2018) POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev 32(13–14):915–928. https://doi.org/10.1101/gad.314815.118
Battifora H (1986) Methods in laboratory investigation. the multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest 55(2):244–248
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinf 18(1):7. https://doi.org/10.1186/s12859-017-1934-z
Sun Y, Zhai C, Chen X, Dong Z, Hou L, Zhou C, Jiang T (2019) Characterization of PD-L1 protein expression and CD8+ tumor-infiltrating lymphocyte density, and their associations with clinical outcome in small-cell lung cancer. Transl Lung Cancer Res 8(6):748–759. https://doi.org/10.21037/tlcr.2019.10.09
Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, Schalper KA (2019) Expression and clinical significance of PD-L1, B7–H3, B7–H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer 7(1):65. https://doi.org/10.1186/s40425-019-0540-1
Berghoff AS, Ricken G, Wilhelm D, Rajky O, Widhalm G, Dieckmann K, Birner P, Bartsch R, Preusser M (2016) Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol 130(1):19–29. https://doi.org/10.1007/s11060-016-2216-8
George J, Lim JS, Jang SJ, Cun Y, Ozretia L, Kong G, Thomas RK (2015) Comprehensive genomic profiles of small cell lung cancer. Nature 524(7563):47–53. https://doi.org/10.1038/nature14664
Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, Nabet BY, Fujimoto J, Solis LM, Lu W, Xi Y, Cardnell RJ, Wang Q, Fabbri G, Cargill KR, Vokes NI, Ramkumar K, Zhang B, Della Corte CM, Robson P, Swisher SG, Roth JA, Glisson BS, Shames DS, Wistuba II, Wang J, Quaranta V, Minna J, Heymach JV, Byers LA (2021) Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 39(3):346–360. https://doi.org/10.1016/j.ccell.2020.12.014
Poirier JT, Gardner EE, Connis N, Moreira AL, de Stanchina E, Hann CL, Rudin CM. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene. 34(48):5869–5878. https://doi.org/10.1038/onc.2015.38
Wooten DJ, Groves SM, Tyson DR, Liu Q, Lim JS, Albert R, Quaranta V (2019) Systems-level network modeling of Small Cell Lung Cancer subtypes identifies master regulators and destabilizers. PLoS Comput Biol 15(10):e1007343. https://doi.org/10.1371/journal.pcbi.1007343
Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, Johnson JE (2016) ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep 16(5):1259–1272. https://doi.org/10.1016/j.celrep.2016.06.081
Zhang W, Girard L, Zhang YA, Haruki T, Papari-Zareei M, Stastny V, Gazdar AF (2018) Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Trans Lung Cancer Res 7(1):32–49. https://doi.org/10.21037/tlcr.2018.02.02
Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, Kim C (2019) STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig 129(10):4350–4364. https://doi.org/10.1172/JCI125413
Chon HJ, Kim H, Noh JH, Yang H, Lee WS, Kong SJ, Kim C (2019) STING signaling is a potential immunotherapeutic target in colorectal cancer. J Cancer 10(20):4932–4938. https://doi.org/10.7150/jca.32806
Gaston J, Cheradame L, Yvonnet V, Deas O, Poupon MF, Judde JG, Goffin V (2016) Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget 7(47):77205–77224. https://doi.org/10.18632/oncotarget.12858
Della Corte CM, Byers LA (2019) Evading the STING: LKB1 loss leads to STING silencing and immune escape in KRAS-mutant lung cancers. Cancer Discov 9(1):16–18. https://doi.org/10.1158/2159-8290.CD-18-1286
Raaby Gammelgaard K, Sandfeld-Paulsen B, Godsk SH, Demuth C, Meldgaard P, Sorensen BS, Jakobsen MR (2021) cGAS-STING pathway expression as a prognostic tool in NSCLC. Translational lung cancer research 10(1):340–354. https://doi.org/10.21037/tlcr-20-524
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, Yin R (2019) Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol 12(1):86. https://doi.org/10.1186/s13045-019-0770-1
Barrett RL, Puré E (2020) Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife 28(9):e57243. https://doi.org/10.7554/eLife.57243
Chen PY, Wei WF, Wu HZ, Fan LS, Wang W (2021) Cancer-associated fibroblast heterogeneity: a factor that cannot be ignored in immune microenvironment remodeling. Front Immunol 8(12):671595. https://doi.org/10.3389/fimmu.2021.671595.PMID:34305902;PMCID:PMC8297463
He Y, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Yu H, Zhou C, Hirsch FR (2017) MHC class II expression in lung cancer. Lung Cancer 112:75–80. https://doi.org/10.1016/j.lungcan.2017.07.030
Kamma H, Yazawa T, Ogata T, Horiguchi H, Iijima T (1991) Expression of MHC class II antigens in human lung cancer cells. Virchows Arch B Cell Pathol Incl Mol Pathol 60(6):407–412. https://doi.org/10.1007/BF02899573
Axelrod ML, Cook RS, Johnson DB, Balko JM (2019) Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res 25(8):2392–2402. https://doi.org/10.1158/1078-0432.CCR-18-3200
Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S (1997) Major histocompatibility complex class IItransfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA 94(13):6886–6891. https://doi.org/10.1073/pnas.94.13.6886
Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, Sanders ME, Lovly CM, Frederick DT, Kelley MC, Richmond A, Irish JM, Shyr Y, Sullivan RJ, Puzanov I, Sosman JA, Balko JM (2016) Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 29(7):10582. https://doi.org/10.1038/ncomms10582.PMID:26822383;PMCID:PMC4740184
Forero A, Li Y, Chen D, Grizzle WE, Updike KL, Merz ND, Downs-Kelly E, Burwell TC, Vaklavas C, Buchsbaum DJ, Myers RM, LoBuglio AF, Varley KE (2016) Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol Res 4(5):390–399. https://doi.org/10.1158/2326-6066.CIR-15-0243
Andres F, Yufeng L, Dongquan C, William EG, Katherine LU, Natalie DM, Katherine EV (2016) Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol Res 4(5):390–399. https://doi.org/10.1158/2326-6066.CIR-15-0243
Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Hodi FS (2018) MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Trans Med 10:450. https://doi.org/10.1126/scitranslmed.aar3342
Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY (2016) Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45(5):1122–1134. https://doi.org/10.1016/j.immuni.2016.10.032
Levine AG, Arvey A, Jin W, Rudensky AY (2014) Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 11:1070–8. https://doi.org/10.1038/ni.3004
Johnson AM, Bullock BL, Neuwelt AJ, Poczobutt JM, Kaspar RE, Li HY, Kwak JW, Hopp K, Weiser-Evans MCM, Heasley LE, Schenk EL, Clambey ET, Nemenoff RA (2020) Cancer cell-intrinsic expression of MHC class II regulates the immune microenvironment and response to anti-PD-1 therapy in lung adenocarcinoma. J Immunol. 204(8):2295–2307. https://doi.org/10.4049/jimmunol.1900778
Cai L, Liu H, Huang F, Fujimoto J, Girard L, Chen J, Li Y, Zhang YA, Deb D, Stastny V, Pozo K, Kuo CS, Jia G, Yang C, Zou W, Alomar A, Huffman K, Papari-Zareei M, Yang L, Drapkin B, Akbay EA, Shames DS, Wistuba II, Wang T, Johnson JE, Xiao G, DeBerardinis RJ, Minna JD, Xie Y, Gazdar AF (2021) Cell-autonomous immune gene expression is repressed in pulmonary neuroendocrine cells and small cell lung cancer. Commun Biol 4(1):314. https://doi.org/10.1038/s42003-021-01842-7
Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, Hong D, Thai TC, Piel B, Han S, Reinhold BB, Duke-Cohan JS, Poitras MJ, Taus LJ, Lizotte PH, Portell A, Quadros V, Santucci AD, Murayama T, Cañadas I, Kitajima S, Akitsu A, Fridrikh M, Watanabe H, Reardon B, Gokhale PC, Paweletz CP, Awad MM, Van Allen EM, Lako A, Wang XT, Chen B, Hong F, Sholl LM, Tolstorukov MY, Pfaff K, Jänne PA, Gjini E, Edwards R, Rodig S, Reinherz EL, Oser MG, Barbie DA (2021) Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. 11(8):1952–1969. https://doi.org/10.1158/2159-8290.CD-20-0913
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT (2008) Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14(16):5220–5227. https://doi.org/10.1158/1078-0432.CCR-08-0133
Acknowledgements
The authors thank the patients and the clinical teams involved. We thank Leslie Rozeboom, UCD, for her assistance in creating TMA blocks. ZL acknowledges funding from the Hungarian National Research, Development and Innovation Office (OTKA #124652 and OTKA #129664). BD acknowledges funding from the Austrian Science Fund (FWF I3522, FWF I3977 and I4677) and from the Hungarian National Research, Development and Innovation Office (KH130356; 2020-1.1.6-JÖVŐ and TKP2021-EGA-33). VL is a recipient of the Bolyai Research Scholarship of the Hungarian Academy of Sciences and the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. ZM was supported by the UNKP-20-3 and UNKP-21-3 New National Excellence Program of the Ministry for Innovation and Technology of Hungary and by the Hungarian Respiratory Society (MPA #2020).
Author information
Authors and Affiliations
Contributions
Conception and design: ZL, DD. Development of methodology: ZL, DD, CR, HY, ZM. Acquisition of data: DD, CR, HY, SLP. Analysis and interpretation of data: DD, ZL, ED, KH, ZM. Administrative, technical or material support: CR, VL, TH, JM, BD, FH, ZM. Study supervision: ZL, DD, BD. Writing and reviewing the manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dora, D., Rivard, C., Yu, H. et al. Protein Expression of immune checkpoints STING and MHCII in small cell lung cancer. Cancer Immunol Immunother 72, 561–578 (2023). https://doi.org/10.1007/s00262-022-03270-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03270-w