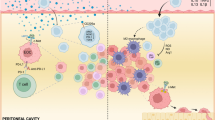
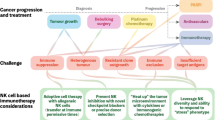
Abstract
We extended our previous observations with other tumor models to study seven ovarian tumor cell lines—OVCAR3, OVCAR4, OVCAR8, SKOV3, Kuramochi, OAW28, and CaOV3. We found that NK cells targeted and killed poorly differentiated OVCAR8 and CAOV3; these two tumor lines express lower MHC-class I and higher CD44 surface receptors. OVCAR3 and OVCAR4 were more resistant to NK cell-mediated cytotoxicity, and SKOV3, Kuramochi and OAW28 had intermediate sensitivity to NK cell-mediated cytotoxicity, likely representing well-differentiated and moderately differentiated ovarian tumor cell lines, respectively. Similar trends were observed for secretion of IFN-γ by the NK cells when co-cultured with different ovarian tumor cell lines. Treatment with both IFN-γ and TNF-α upregulated MHC-class I in all ovarian tumor cell lines and resulted in tumor resistance to NK cell-mediated cytotoxicity and decreased secretion of IFN-γ in co-cultures of NK cells with tumors cells with the exception of OVCAR8 and CAOV3 which did not upregulate MHC-class I and remained sensitive to NK cell-mediated cytotoxicity and increased secretion of IFN-γ when co-cultured with NK cells. Similarly, treatment with NK cell supernatants induced resistance to NK cell-mediated cytotoxicity in OVCAR4 but not in OVCAR8, and the resistance to killing was correlated with the increased surface expression of MHC-class I in OVCAR4 but not in OVCAR8. In addition, OVCAR4 was found to be carboplatin sensitive before and after treatment with IFN-γ and NK cell supernatants, whereas OVCAR8 remained carboplatin resistant with and without treatment with IFN-γ and NK cell supernatants. Overall, sensitivity to NK cell-mediated killing correlated with the levels of tumor differentiation and aggressiveness, and more importantly, poorly differentiated ovarian tumors were unable to upregulate MHC-class I under the activating conditions for MHC-class I, a feature that was not seen in other tumor models and may likely be specific to ovarian tumors. Such tumors may also pose a significant challenge in elimination by the T cells; however, NK cells are capable of targeting such tumors and can be exploited to eliminate these tumors in immunotherapeutic strategies.






Similar content being viewed by others
References
Beaufort CM et al (2014) Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS ONE 9(9):e103988
Haley J et al (2016) Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget 7(22):32810–32820
Perera Molligoda Arachchige AS (2021) Human NK cells: from development to effector functions. Innate Immun 27(3):212–229
Poli A et al (2009) CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126(4):458–465
Bui VT et al (2015) Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol 6:576
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Tseng HC et al (2014) Induction of split anergy conditions natural killer cells to promote differentiation of stem cells through cell-cell contact and secreted factors. Front Immunol 5:269
Hersey P et al (1979) Low natural-killer-cell activity in familial melanoma patients and their relatives. Br J Cancer 40(1):113–122
Kuss I et al (1999) Clinical significance of decreased zeta chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res 5(2):329–334
Guillerey C (2020) NK Cells in the tumor microenvironment. Adv Exp Med Biol 1273:69–90
Lee JC et al (2004) Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 172(12):7335–7340
Imai K et al (2000) Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356(9244):1795–1799
Jewett A et al (2008) Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab’)2 fragment of anti-CD16 antibody. Cancer Immunol Immunother 57(7):1053–1066
Pietra G et al (2012) Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 72(6):1407–1415
Hershey P et al (1978) Relationship of cell-mediated cytotoxicity against melanoma cells to prognosis in melanoma patients. Br J Cancer 37(4):505–513
Lai P et al (1996) Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res 2(1):161–173
Burke S et al (2010) New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol 31(9):339–345
Brittenden J et al (1996) Natural killer cells and cancer. Cancer 77(7):1226–1243
Patankar MS et al (2005) Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol 99(3):704–713
Gubbels JA et al (2010) MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer 9:11
Gubbels JA et al (2006) Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 5(1):50
Johnson SW et al (1997) Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res 57(5):850–856
Mitra AK et al (2015) In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol 138(2):372–377
Gottschalk N et al (2012) Monocytes and the 38kDa-antigen of mycobacterium tuberculosis modulate natural killer cell activity and their cytolysis directed against ovarian cancer cell lines. BMC Cancer 12:451
Gao Y et al (2015) Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 6(11):9313–9326
Natoli M et al (2020) Human ovarian cancer intrinsic mechanisms regulate lymphocyte activation in response to immune checkpoint blockade. Cancer Immunol Immunother 69(8):1391–1401
Fogh J, Fogh JM, Orfeo T (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59(1):221–226
Sun Y et al (2018) Natural killer cells inhibit metastasis of ovarian carcinoma cells and show therapeutic effects in a murine model of ovarian cancer. Exp Ther Med 16(2):1071–1078
Hoogstad-van Evert JS et al (2020) Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol Oncol 157(3):810–816
Gonzalez VD et al (2020) High-grade serous ovarian tumor cells modulate NK cell function to create an immune-tolerant microenvironment. bioRxiv 9:361
Cannistra SA et al (1995) Functional heterogeneity of CD44 molecules in ovarian cancer cell lines. Clin Cancer Res 1(3):333–342
Cannistra SA et al (1993) Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 53(16):3830–3838
Kaur K et al (2019) Probiotic-treated super-charged NK cells efficiently clear poorly differentiated pancreatic tumors in Hu-BLT mice. Cancers (Basel) 12(1):63
Kozlowska AK et al (2017) Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer 8(4):537–554
Bui VT et al (2015) Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. https://doi.org/10.3389/fimmu.2015.00576
Jewett A, Man Y-G, Tseng H-C (2013) Dual functions of natural killer cells in selection and differentiation of stem cells; role in regulation of inflammation and regeneration of tissues. J Cancer 4(1):12–24
Jewett A, Bonavida B (1996) Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol 156(3):907–915
Tseng H-C et al (2010) Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE 5(7):e11590
Kaur K et al (2018) Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology 7(5):e1426518
Kaur K et al (2017) Novel strategy to expand super-charged NK Cells with significant potential to lyse and differentiate cancer stem cells: differences in NK expansion and function between healthy and cancer patients. Front Immunol 8:297
Bui VT et al (2015) Augmented IFN-gamma and TNF-alpha induced by probiotic bacteria in NK Cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol 6:576
Tseng HC et al (2015) Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: role in osteoclast-mediated NK cell activation. Oncotarget 6(24):20002–20025
Wagstaff AJ et al (1989) Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs 37(2):162–190
Jewett A et al (2020) Multiple defects of natural killer cells in cancer patients: anarchy, dysregulated systemic immunity, and immunosuppression in metastatic cancer. Crit Rev Immunol 40(2):93–133
Nersesian S et al (2019) Naturally killing the silent killer: NK cell-based immunotherapy for ovarian cancer. Front Immunol 10:1782–1782
Lutgendorf SK et al (2005) Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol 23(28):7105–7113
Wicks IP et al (1992) The effect of cytokines on the expression of MHC antigens and ICAM-1 by normal and transformed synoviocytes. Autoimmunity 12(1):13–19
Galluzzi L et al (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31(15):1869–1883
Halon A et al (2011) Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res 31(2):711–718
Cornel AM, Mimpen IL, Nierkens S (2020) MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel) 12(7):1760
Jewett A, Cavalcanti M, Bonavida B (1997) Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol 159(10):4815–4822
Jewett A, Bonavida B (1995) Interferon-alpha activates cytotoxic function but inhibits interleukin-2-mediated proliferation and tumor necrosis factor-alpha secretion by immature human natural killer cells. J Clin Immunol 15(1):35–44
Jewett A et al (2003) Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: effect on the function of natural killer cells. Hum Immunol 64(5):505–520
Acknowledgements
The authors are grateful to funding agencies and donors for supporting the work.
Author information
Authors and Affiliations
Contributions
NC, SHY, and PCC generated data, reviewed, and edited the article. KK analyzed data, prepared figures, wrote, reviewed, and edited the article. NA and GD provided tumor samples and technical help. SG reviewed and edited the paper. AJ and SM oversaw the studies, conceptualization of the article, reviewed and edited the article, and acquired funding.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chovatiya, N., Kaur, K., Huerta-Yepez, S. et al. Inability of ovarian cancers to upregulate their MHC-class I surface expression marks their aggressiveness and increased susceptibility to NK cell-mediated cytotoxicity. Cancer Immunol Immunother 71, 2929–2941 (2022). https://doi.org/10.1007/s00262-022-03192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03192-7