Abstract
Background
Tumor-infiltrating CD8 cells and expression of programmed cell death ligand 1 (PD-L1) are immune checkpoint markers in patients with hepatocellular carcinoma (HCC). We aimed to determine the ability of preoperative gadoxetic acid-enhanced magnetic resonance imaging (MRI) findings to predict CD8 cell density and PD-L1 expression in HCC.
Methods
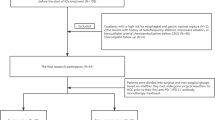
A total of 120 patients with HCC who underwent 3.0-T gadoxetic acid-enhanced MRI before curative resection from January 2016 to June 2020 were enrolled and divided into a training set (n = 84) and a testing set (n = 36). Thirty-four patients with advanced stage HCC who received anti-PD-1 inhibitor between January 2017 and April 2020 and underwent pretreated gadoxetic acid-enhanced MRI scans were enrolled in an independent validation set. PD-L1 expression and CD8 cell infiltration were assessed with immunohistochemical staining, respectively. Two radiologists blinded to pathology results evaluated the pretreated MR features in consensus. Logistic regression and the receiver operating characteristic curve (ROC) analyses were used to determine the value of image features to predict high CD8 cell density, PD-L1 positivity and the combination of high CD8 cell density and PD-L1 positivity in HCC in the training set and validated the findings in the testing set. The associations of MRI predictors with the objective response to immunotherapy were assessed in the independent validation.
Results
In the training set, the independent MRI predictors were irregular tumor margin (ITM, P = 0.008) and peritumoral low signal intensity (PLSI) on hepatobiliary phase (HBP) images (P < 0.001) for PD-L1 positivity, absence of an enhancing capsule (AEC, P = 0.001) and PLSI on HBP images (P = 0.025) for high CD8 cell density, and PLSI on HBP images (P = 0.001) and ITM (P = 0.023) for the both. The area under the curves (AUCs) of the predictive models for evaluating PD-L1 positivity, high CD8 cell density and the combination of high CD8 cell density and PD-L1 positivity were 0.810 and 0.809, 0.740 and 0.728, and 0.809 and 0.874 in the training and testing set, respectively. The objective response was demonstrated to be associated with the combination of PLSI on HBP images and ITM (PHI, P = 0.004), and the combination of PLSI on HBP images and AEC (PHA, P = 0.012) in the independent validation set.
Conclusions
Pretreated MRI features have the potential to identify patients with HCC in an immune-activated state and predict outcomes of immunotherapy.
Trial registration
The study was retrospectively registered on March 5, 2020 with registration no. [2020] 02–012-01.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- AEC:
-
Absence of enhancing capsule
- AP:
-
Arterial phase
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- HBP:
-
Hepatobiliary phase
- HCC:
-
Hepatocellular carcinoma
- ITM:
-
Irregular tumor margin
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MRI:
-
Magnetic resonance imaging
- NLR:
-
Negative likelihood ratio
- OR:
-
Odds ratio
- PD-1:
-
Programmed cell death protein-1
- PD-L1:
-
Programmed cell death ligand 1
- PHA:
-
The combination of PLSI on HBP images and AEC
- PHI:
-
The combination of PLSI on HBP images and ITM
- PLR:
-
Positive likelihood ratio
- PLSI:
-
Peritumoral low signal intensity
- PVP:
-
Portal venous phase
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- ROC:
-
Receiver operating characteristic curve
- TP:
-
Transitional phase
References
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380(15):1450–1462. https://doi.org/10.1056/NEJMra1713263
Lee HW, Cho KJ, Park JY (2020) Current status and future direction of immunotherapy in Hepatocellular Carcinoma: what do the data suggest. Immune Netw 20(1):e11. https://doi.org/10.4110/in.2020.20.e11
Rebouissou S, Nault JC (2020) Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 72(2):215–229. https://doi.org/10.1016/j.jhep.2019.08.017
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355. https://doi.org/10.1126/science.aar4060
Mahipal A, Tella SH, Kommalapati A, Lim A, Kim R (2019) Immunotherapy in Hepatocellular Carcinoma: is there a light at the end of the tunnel. Cancers (Basel). https://doi.org/10.3390/cancers11081078
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M, KEYNOTE-224 investigators (2018) Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19(7):940–952. https://doi.org/10.1016/S1470-2045(18)30351-6
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Rd WTH, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Jiang Y, Han QJ, Zhang J (2019) Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol 25(25):3151–3167. https://doi.org/10.3748/wjg.v25.i25.3151
Hegde PS, Karanikas V, Evers S (2016) The where, the When, and the how of Immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res 22(8):1865–1874. https://doi.org/10.1158/1078-0432.CCR-15-1507
Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, Hollebecque A, Scoazec JY, Marabelle A, Massard C, Soria JC, Robert C, Paragios N, Deutsch E, Ferté C (2018) A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. https://doi.org/10.1016/S1470-2045(18)30413-3
Chang H, Jung W, Kim A, Kim HK, Kim WB, Kim JH, Kim BH (2017) Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1, and cytotoxic T lymphocyte-associated molecule-4 in hepatocellular carcinoma. APMIS 125(8):690–698. https://doi.org/10.1111/apm.12703
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. https://doi.org/10.1038/nature14011
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Tang C, Hobbs B, Amer A, Li X, Behrens C, Canales JR, Cuentas EP, Villalobos P, Fried D, Chang JY, Hong DS, Welsh JW, Sepesi B, Court L, Wistuba II, Koay EJ (2018) Development of an immune-pathology informed radiomics model for non-small cell lung cancer. Sci Rep 8(1):1922. https://doi.org/10.1038/s41598-018-20471-5
Chen S, Feng S, Wei J, Liu F, Li B, Li X, Hou Y, Gu D, Tang M, Xiao H, Jia Y, Peng S, Tian J, Kuang M (2019) Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol 29(8):4177–4187. https://doi.org/10.1007/s00330-018-5986-x
Liao H, Zhang Z, Chen J, Liao M, Xu L, Wu Z, Yuan K, Song B, Zeng Y (2019) Preoperative radiomic approach to evaluate tumor-infiltrating CD8+ T cells in Hepatocellular Carcinoma patients using contrast-enhanced computed tomography. Ann Surg Oncol 26(13):4537–4547. https://doi.org/10.1245/s10434-019-07815-9
Hectors SJ, Lewis S, Besa C, King MJ, Said D, Putra J, Ward S, Higashi T, Thung S, Yao S, Laface I, Schwartz M, Gnjatic S, Merad M, Hoshida Y, Taouli B (2020) MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. https://doi.org/10.1007/s00330-020-06675-2
Saha A, Harowicz MR, Mazurowski MA (2018) Breast cancer MRI radiomics: an overview of algorithmic features and impact of inter-reader variability in annotating tumors. Med Phys 45(7):3076–3085. https://doi.org/10.1002/mp.12925
Cho ES, Choi JY (2015) MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol 16(3):449–464. https://doi.org/10.3348/kjr.2015.16.3.449
Zhang L, Kuang S, Chen J, Zhang Y, Zhao B, Peng H, Xiao Y, Fowler K, Wang J, Sirlin CB (2019) The role of preoperative dynamic contrast-enhanced 3.0-T MR imaging in predicting early recurrence in patients with early-stage hepatocellular carcinomas after curative resection. Front Oncol 9:1336. https://doi.org/10.3389/fonc.2019.01336
Chen J, Zhou J, Kuang S, Zhang Y, Xie S, He B, Deng Y, Yang H, Shan Q, Wu J, Sirlin CB, Wang J (2019) Liver imaging reporting and data system category 5: MRI predictors of microvascular invasion and recurrence after hepatectomy for Hepatocellular Carcinoma. AJR Am J Roentgenol 213(4):821–830. https://doi.org/10.2214/AJR.19.21168
Huang K, Dong Z, Cai H, Huang M, Peng Z, Xu L, Jia Y, Song C, Li ZP, Feng ST (2019) Imaging biomarkers for well and moderate hepatocellular carcinoma: preoperative magnetic resonance image and histopathological correlation. BMC Cancer 19(1):364. https://doi.org/10.1186/s12885-019-5574-8
Chou YC, Lao IH, Hsieh PL, Su YY, Mak CW, Sun DP, Sheu MJ, Kuo HT, Chen TJ, Ho CH, Kuo YT (2019) Gadoxetic acid-enhanced magnetic resonance imaging can predict the pathologic stage of solitary hepatocellular carcinoma. World J Gastroenterol 25(21):2636–2649. https://doi.org/10.3748/wjg.v25.i21.2636
Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, Fujimoto J, Yu H, Anders R, Kowalewski A, Rivard C, Rehman J, Batenchuk C, Burns V, Hirsch FR, Wistuba II (2017) A Prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 3(8):1051–1058. https://doi.org/10.1001/jamaoncol.2017.0013
Pang X, Qian J, Jin H, Zhang L, Lin L, Wang Y, Lei Y, Zhou Z, Li M, Zhang H (2020) Durable benefit from immunotherapy and accompanied lupus erythematosus in pancreatic adenocarcinoma with DNA repair deficiency. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000463
Xie QK, Zhao YJ, Pan T, Lyu N, Mu LW, Li SL, Shi MD, Zhang ZF, Zhou PH, Zhao M (2016) Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology 5(7):e1181252. https://doi.org/10.1080/2162402X.2016.1181252
Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R, Li W, Wu P (2019) The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother 68(3):353–363. https://doi.org/10.1007/s00262-018-2271-4
Cerny M, Chernyak V, Olivié D, Billiard JS, Murphy-Lavallée J, Kielar AZ, Elsayes KM, Bourque L, Hooker JC, Sirlin CB, Tang A (2018) LI-RADS version 2018 ancillary features at MRI. Radiographics 38(7):1973–2001. https://doi.org/10.1148/rg.2018180052
Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB (2018) Liver imaging reporting and data system (LI-RADS) version 2018: imaging of Hepatocellular Carcinoma in at-risk patients. Radiology 289(3):816–830. https://doi.org/10.1148/radiol.2018181494
Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, Lee BR (2018) Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive Hepatocellular Carcinoma: a retrospective observational study. Radiology 286(3):897–908. https://doi.org/10.1148/radiol.2017162846
Lee S, Kim SH, Lee JE, Sinn DH, Park CK (2017) preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 67(3):526–534. https://doi.org/10.1016/j.jhep.2017.04.02429
Iannaccone R, Piacentini F, Murakami T, Paradis V, Belghiti J, Hori M, Kim T, Durand F, Wakasa K, Monden M, Nakamura H, Passariello R, Vilgrain V (2007) Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: helical CT and MR imaging findings with clinical-pathologic comparison. Radiology 243(2):422–430. https://doi.org/10.1148/radiol.2432051244
Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE (2012) Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging 35(3):629–634. https://doi.org/10.1002/jmri.22876
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 19(14):3936–3943. https://doi.org/10.1158/1078-0432.CCR-13-0895
Li JH, Ma WJ, Wang GG, Jiang X, Chen X, Wu L, Liu ZS, Zeng XT, Zhou FL, Yuan YF (2018) Clinicopathologic significance and prognostic value of programmed cell death ligand 1 (PD-L1) in patients with Hepatocellular Carcinoma: a meta-analysis. Front Immunol 9:2077. https://doi.org/10.3389/fimmu.2018.02077
Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS (2012) MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int 32(3):430–440. https://doi.org/10.1111/j.1478-3231.2011.02640.x
Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW (2009) Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI. Eur Radiol 19(7):1744–1751. https://doi.org/10.1007/s00330-009-1331-8
Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, Nishie A, Hirakawa M, Ushijima Y, Okamoto D, Taketomi A, Honda H (2009) Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 250(2):435–443. https://doi.org/10.1148/radiol.2501071702
Dioguardi Burgio M, Picone D, Cabibbo G, Midiri M, Lagalla R, Brancatelli G (2016) MR-imaging features of hepatocellular carcinoma capsule appearance in cirrhotic liver: comparison of gadoxetic acid and gadobenate dimeglumine. Abdom Radiol (NY) 41(8):1546–1554. https://doi.org/10.1007/s00261-016-0726-7
Santillan C, Fowler K, Kono Y, Chernyak V (2018) LI-RADS major features: CT, MRI with extracellular agents, and MRI with hepatobiliary agents. Abdom Radiol (NY) 43(1):75–81. https://doi.org/10.1007/s00261-017-1291-4
Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Abastado JP (2012) Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 61(3):427–438. https://doi.org/10.1136/gutjnl-2011-300509
Ramzan M, Sturm N, Decaens T, Bioulac-Sage P, Bancel B, Merle P, Tran Van Nhieu J, Slama R, Letoublon C, Zarski JP, Jouvin-Marche E, Marche PN, Leroy V (2016) Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int 36(3):434–444. https://doi.org/10.1111/liv.12927
Ihling C, Naughton B, Zhang Y, Rolfe PA, Frick-Krieger E, Terracciano LM, Dussault I (2019) Observational study of PD-L1, TGF-β, and immune cell infiltrates in Hepatocellular Carcinoma. Front Med (Lausanne) 6:15. https://doi.org/10.3389/fmed.2019.00015
Huang CY, Wang Y, Luo GY, Han F, Li YQ, Zhou ZG, Xu GL (2017) Relationship between PD-L1 expression and CD8+ T-cell immune responses in Hepatocellular Carcinoma. J Immunother 40(9):323–333. https://doi.org/10.1097/CJI.0000000000000187
Funding
This study has received funding by National Natural Science Foundation of China grant 91959118 (JW); Science and Technology Program of Guangzhou, China grant number 201704020016 (JW); The Key Research and Development Program of Guangdong Province, 2019B020235002 (JW); SKY Radiology Department International Medical Research Foundation of China Z-2014–07-1912–15 (JW); Guangdong Basic and Applied Basic Research Foundation, 2021A1515010582 (JW) and Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-sen University YHJH201901 (JW).
Author information
Authors and Affiliations
Contributions
Jin Wang, Lin Sun, and Luwen Mu participated in the conception and design of this study. Lin Sun, Luwen Mu, Wenjie Tang, Linqi Zhang, Sidong Xie, and Jingbiao Chen contributed to the selecting of patients and acquisition and analysis and review of the data. Jing Zhou performed all histopathological analyses and participated in the writing of the manuscript. Lin Sun and Luwen Mu analyzed the data and wrote the manuscript. Jin Wang supervised the drafting of the manuscript. All authors critically reviewed each draft and approved the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, as reflected by the approval of the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Date: March 5, 2020/No. [2020] 02–012-01). Patients gave their written consent to usage of their tumor specimen.
Consent to participate
All patients gave written informed consent to the use of their tumors and their data for research and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, L., Mu, L., Zhou, J. et al. Imaging features of gadoxetic acid-enhanced MR imaging for evaluation of tumor-infiltrating CD8 cells and PD-L1 expression in hepatocellular carcinoma. Cancer Immunol Immunother 71, 25–38 (2022). https://doi.org/10.1007/s00262-021-02957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02957-w