Abstract
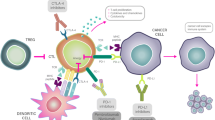
Triple-negative breast cancer (TNBC) is defined by a lack of expression of both estrogen (ER) and progesterone (PgR) receptors as well as human epidermal growth factor receptor 2 (HER2) and is associated with poor prognosis. Moreover, the systemic treatment options are limited. However, the TNBC is more likely than other breast cancer subtypes to benefit from immune checkpoint blockade therapy due to its higher immunogenicity, higher enrichment by tumour-infiltrating lymphocytes (TILs), and higher levels of programmed cell death ligand 1 (PD-L1) expression. Thus far, atezolizumab was approved in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic TNBC whose tumours express PD-L1. Currently, it seems that PD-L1-positive subgroup will potentially benefit the most from the immune checkpoint inhibitor (ICI) treatment. Moreover, it seems that better results are seen when an ICI is given as first-line treatment than when an ICI is given in later lines of treatment for advanced TNBC/metastatic TNBC. Recently, pembrolizumab has demonstrated promising results in early-stage TNBC what can lead in near future to its approval in (neo)adjuvant setting. This review summarizes the development and highlights recent advances of the atezolizumab and pembrolizumab in early and advanced/metastatic TNBC.
Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- AKT:
-
Protein kinase B
- Anti-PD-1:
-
Anti-programmed death receptor 1
- ASCO:
-
American Society of Clinical Oncology
- aTNBC:
-
Advanced triple-negative breast cancer
- BC:
-
Breast cancer
- BRCA:
-
Breast cancer gene
- CPS:
-
Combined positive score
- DFS:
-
Disease-free survival
- DLTs:
-
Dose-limiting toxicities
- EFS:
-
Event-free survival
- ER:
-
Estrogen receptor
- FDA:
-
US Food and Drug Administration
- HER2:
-
Human epidermal growth factor receptor 2
- ICI:
-
Immune checkpoint inhibitor
- ICs:
-
Immune cells
- ITT:
-
Intention-to-treat
- LDH:
-
Lactate dehydrogenase
- MEK:
-
Mitogen-activated protein kinase kinase
- mTNBC:
-
Metastatic triple-negative breast cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- PD-L1:
-
Programmed cell death ligand 1
- PFS:
-
Progression-free survival
- PgR:
-
Progesterone receptor
- TILs:
-
Tumour-infiltrating lymphocytes
- TMB:
-
Tumour mutational burden
- TNBC:
-
Triple-negative breast cancer
- TRAEs:
-
Treatment-related adverse events
References
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Haffty BG, Yang Q, Reiss M et al (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24:5652–5657
Mittendorf EA, Philips AV, MericBernstam F et al (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2:361–370
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50
Luen S, Virassamy B, Savas P et al (2016) The genomic landscape of breast cancer and its interaction with host immunity. Breast 29:241–250
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–5464
Cardoso F, Costa A, Senkus E et al (2017) 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 31:244–259
Garrido-Castro AC, Lin NU, Polyak K (2019) Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 9:176–198
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121
FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast cancer. FDA U.S. Food and Drug. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative. Accessed 16 May 2020
Schmid P, Rugo HS, Adams S et al (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:44–59
Schmid P, Salgado R, Park YH et al (2020) Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol 31:569–581
Nanda R, Liu MC, Yau C et al (2020) Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 2020:e196650. https://doi.org/10.1001/jamaoncol.2019.6650
Gianni L, Huang C, Egle D, et al (2019) Pathologic complete response to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. 2019 San Antonio Breast Cancer Symposium. Abstract GS3–04. Presented December 12, 2019
Roche’s Tecentriq in combination with chemotherapy (including Abraxane) meets primary endpoint of improved pathological complete response, regardless of PD-L1 status, as initial treatment for people with early triple-negative breast cancer. Roche. https://www.roche.com/media/releases/med-cor-2020-06-18.htm. Accessed 10 August 2020
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810–821
von Minckwitz G, Untch M, Blohmer J-U et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Berruti A, Amoroso V, Gallo F et al (2014) Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 32:3883–3891
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 10 August 2020
Emens LA, Cruz C, Eder JP et al (2019) Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 5:74–82
Nanda R, Chow LQM, Dees EC et al (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34:2460–2467
Adams S, Loi S, Toppmeyer D et al (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30:405–411
Adams S, Schmid P, Rugo HS et al (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30:397–404
Cortés J, Lipatov O, Im SA et al (2019) KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol 30(Suppl 5):v859–860
Winer EP, Lipatov O, Im S-A et al (2020) Association of tumor mutational burden (TMB) and clinical outcomes with pembrolizumab (pembro) versus chemotherapy (chemo) in patients with metastatic triple-negative breast cancer (mTNBC) from KEYNOTE-119. J Clin Oncol 38(suppl):1013. https://doi.org/10.1200/JCO.2020.38.15_suppl.1013
Adams S, Diamond JR, Hamilton E et al (2019) Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-Year survival follow-up: a phase 1b clinical trial. JAMA Oncol 5:334–342
Tolaney SM, Kalinsky K, Kaklamani VG et al (2020) A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). J Clin Oncol 38(suppl):1015. https://doi.org/10.1200/JCO.2020.38.15_suppl.1015
Vinayak S, Tolaney SM, Schwartzberg L et al (2019) Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol 5:1132–1140
Shah AN, Flaum L, Helenowski I et al (2020) Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2−negative endocrine-refractory metastatic breast cancer. J Immunother Cancer 8:e000173. https://doi.org/10.1136/jitc-2019-000173
Page DB, Chun B, Pucilowska J et al (2019) Pembrolizumab (pembro) with paclitaxel (Taxol) or capecitabine (CAPE) as early treatment of metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 37:1015
Ho AY, Barker CA, Arnold BB et al (2020) A Phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 126:850–860
Kok M, Voorwerk L, Horlings H et al (2018) Adaptive phase II randomized trial of nivolumab after induction treatment in triple negative breast cancer (TONIC trial): final response data stage I and first translational data. J Clin Oncol 36(suppl):1012
O'Shaughnessy J, Moroose RL, Babu S et al (2020) Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J Clin Oncol 38(suppl):1014. https://doi.org/10.1200/JCO.2020.38.15_suppl.1014
Brufsky A, Kim SB, Zvirbule Z et al (2019) Phase II COLET study: Atezolizumab (A) + cobimetinib (C) + paclitaxel (P)/nab-paclitaxel (nP) as first-line (1L) treatment (tx) for patients (pts) with locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 37(Suppl 15):1013
Iwata H, Inoue K, Kaneko K et al (2019) Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn J Clin Oncol 49:1083–1091
Roche provides update on Phase III study of Tecentriq in combination with paclitaxel for people with metastatic triple-negative breast cancer. Roche. https://www.roche.com/investors/updates/inv-update-2020-08-06.htm. Accessed 10 August 2020
Cortés J, Cescon DW, Rugo HS et al (2020) KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol 38(Suppl. 15):1000
EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000977-62/GB. Accessed 26 May 2020
Schmid P, Loirat D, Savas P et al (2019) Phase Ib study evaluating a triplet combination of ipatasertib (IPAT), atezolizumab (atezo), and paclitaxel (PAC) or nab-PAC as first-line (1L) therapy for locally advanced/metastatic triple-negative breast cancer (TNBC). Cancer Res 79(13 Suppl):Abstract CT049
Author information
Authors and Affiliations
Contributions
Dorota Kwapisz—conception and design/idea for the article, acquisition of data/literature search, analysis and interpretation of data, wrote the paper, critically revised the work, approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author has no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother 70, 607–617 (2021). https://doi.org/10.1007/s00262-020-02736-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02736-z