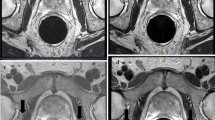
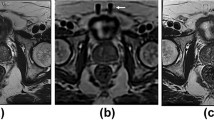
Abstract
Purpose
To compare a previous model-based image reconstruction (MBIR) with a newly developed deep learning (DL)-based image reconstruction for providing improved signal-to-noise ratio (SNR) in high through-plane resolution (1 mm) T2-weighted spin-echo (T2SE) prostate MRI.
Methods
Large-area contrast and high-contrast spatial resolution of the reconstruction methods were assessed quantitatively in experimental phantom studies. The methods were next evaluated radiologically in 17 subjects at 3.0 Tesla for whom prostate MRI was clinically indicated. For each subject, the axial T2SE raw data were directed to MBIR and to the DL reconstruction at three vendor-provided levels: (L)ow, (M)edium, and (H)igh. Thin-slice images from the four reconstructions were compared using evaluation criteria related to SNR, sharpness, contrast fidelity, and reviewer preference. Results were compared using the Wilcoxon signed-rank test using Bonferroni correction, and inter-reader comparisons were done using the Cohen and Krippendorf tests.
Results
Baseline contrast and resolution in phantom studies were equivalent for all four reconstruction pathways as desired. In vivo, all three DL levels (L, M, H) provided improved SNR versus MBIR. For virtually, all other evaluation criteria DL L and M were superior to MBIR. DL L and M were evaluated as superior to DL H in fidelity of contrast. For 44 of the 51 evaluations, the DL M reconstruction was preferred.
Conclusion
The deep learning reconstruction method provides significant SNR improvement in thin-slice (1 mm) T2SE images of the prostate while retaining image contrast. However, if taken to too high a level (DL High), both radiological sharpness and fidelity of contrast diminish.
Graphical abstract









Similar content being viewed by others
References
Crooks LE, Ortendahl DA, Kaufman L, et al. Clinical efficacy of nuclear magnetic resonance imaging. Radiology. 1983;146:123-8.
Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823-33.
Mugler JP, Bao S, Mulkern RV, et al. Optimized single slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216:891-9.
Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55:1030-7.
Gold GE, Busse RF, Beehler C, et al. Isotropic MRI of the knee with 3D fast spin-echo extended echo-train acquistiion (XETA): initial experience. AJR. 2007;188:1287-93.
Rosenkrantz AB, Neil J, Kong X, et al. Prostate cancer: comparison of 3D T2-weighted with conventional 2D T2-weighted imaging for image quality and tumor detection. Am J Roentgenol. 2010;194:446-52.
Aiken AH, Mukherjee P, Green AJ, Glastonbury CM. MR imaging of optic neuropathy with extended echo-train acquisition fluid-attenuated inversion recovery. Am J Neuroradiol. 2011;32:301-5.
Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Img. 2014;39:745-67.
Srinivasan S, Wu HH, Sung K, Margolis DJA, Ennis DB. Fast 3D T2-weighted imaging usign variable flip angle transition into driven equilibrium (3D T2-TIDE) balanced SSFP for prostate imaging at 3T. Magn Reson Med. 2015;74:442-51.
Vidya Shankar R, Roccia E, Cruz G, et al. Accelerated 3D T2w-imaging of the prostate with 1-millimeter isotropic resolution in less than 3 minutes. Magn Reson Med. 2019;82:721-31.
Wilman AH, Riederer SJ. Improved centric phase encoding orders for three dimensional magnetization prepared MR angiography. Magn Reson Med. 1996;36:384-92.
Choi MH, Kim DH, Lee YJ, Rha SE, Lee JY. Image features of the PI-RADS for predicting extraprostatic extension of prostate cancer: systematic review and meta-analysis. Insights into Imaging. 2023;14:77.
Karademir I, Shen D, Peng Y, et al. Prostate volumes derived from MRI and volume-adjusted serum prostate-specific antigen: correlation with Gleason score of prostate cancer. 2013;201:1041-8.
Vargas HA, Akin O, Shukla-Dave A, et al. Performance characteristics of MR imaging in the evaluation of clincally low-risk prostate cancer: a prospective study. Radiology. 2012;265:478-87.
Greenspan H, Oz G, Kiryati N, Peled S. MRI inter-slice reconstruction using super-resolution. Magn Reson Img. 2002;20:437-46.
Shilling RZ, Robbie TQ, Bailloeul T, Mewes K, Mersereau RM, Brummer ME. A super-resolution framework for 3-D high-resolution and high-contrast imaging using 2-D multislice MRI. IEEE Trans Med Img. 2009;28:633-44.
Aganj I, Lenglet C, Yacoub E, Sapiro G, Harel N. A 3D wavelet fusion approach for the reconstruction of isotropic-resolution MR images from orthogonal anisotropic resolution scans. Magn Reson Med. 2012;67:1167-72.
Plenge E, Poot DHJ, Bernsen M, et al. Super-resolution methods in MRI: can they improve the trade-off between resolution, signal-to-noise ratio, and acquisition time? Magn Reson Med. 2012;68:1983-93.
Gholipour A, Afacan O, Aganj I, et al. Super-resolution reconstruction in frequency, image, and wavelet domains to reduce through-plane partial voluming in MRI. Med Phys. 2015;42:6919-32.
Okanovic M, Hillig B, Breuer F, Jakob P, Blaimer M. Time-of-flight MR-angiography with a helical trajectory and slice-super-resolution reconstruction. Magn Reson Med. 2018;80:1812-23.
Kargar S, Borisch EA, Froemming AT, et al. Use of kZ-space for high through-plane resolution in multi-slice MRI: application to prostate. Magn Reson Med. 2019;81:3691-704.
Borisch EA, Grimm RC, Froemming AT, Kawashima A, Trzasko JD, Riederer SJ. Model-based image reconstruction with wavelet sparsity regularization for through-plane resolution restoration in T2-weighted spin-echo prostate MRI. Magn Reson Med. 2023;89:454-68.
Wang X, Ma J, Bhosale P, et al. Novel deep learning-based noise reduction technique for prostate magnetic resonance imaging. Abdom Radiol. 2021;46:3378-86.
Gassenmaier S, Afat S, Nickel D, Mostapha M, Hermann J, Othman AE. Deep learning-accelerated T2-weighted imaging of the prostate: reduction of acquisition time and improvement of image quality. Eur J Radiol. 2021;137:109600.
Park JC, Park EJ, Park MY, Kim M, Kim JK. Fast T2-weighted imaging with deep learning-based reconstruction: evaluation of image quality and diagnostic performance in patients undergoing radical prostatectomy. J Magn Reson Img. 2022;55:1734-44.
Kelleher CB, Macdonald J, Jaffe TA, et al. A faster prostate MRI: comparing a novel denoised, single-average T2 sequence to the conventional multiaverage T2 sequence regarding lesion detection and PI-RADS score assessment. J Magn Reson Img. 2023;58:620-9.
Tong A, Bagga B, Petrocelli R, et al. Comparison of a deep learning-accelerated vs. conventional T2-weighted sequence in biparametric MRI of the prostate. J Magn Reson Img. 2023;58:1055–64.
Kargar S, Borisch EA, Froemming AT, et al. Modified acquisition strategy for reduced motion artifact in super resolution T2FSE multislice MRI: application to prostate. Magn Reson Med. 2020;84:2537-50.
Lebel RM. Performance characteristization of a novel deep learning-based MR image reconstruction pipeline. arXiv. 2020;arXiv:2008.06559.
Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urology. 2019;76:340-51.
Keenan KE, Stupic KF, Boss MA, et al. Multi-site, multi-vendor comparison of T1 measurement using ISMRM/NIST system phantom. 24th Annual Mtg, ISMRM. Singapore2016; p. 3290.
Senn S, Stevens L, Chaturvedi N. Repeated measures in clinical trials: simple strategies for analysis using summary measures. Statistics in Med. 2000;19:861-77.
Schober P, Vetter TR. Repeated measures designs and analysis of longitudinal data: if a first you do not succeed - try, try again. Anesth & Analg. 2018;127:569-74.
Rosner B. Fundamentals of Biostatistics. Sixth ed. Belmont CA: Thomson, 2006; p. 868.
Cohen J. A coefficeint of agreement for nominal scales. Educ Psych Meas. 1960;20:37-46.
Krippendorf K. Content analysis: an introduction to its methodology. Thousand Oaks CA, 2013: 221–250.
Lee CH. Quantitative T2-mapping using MRI for detection of prostate malignancy: a systematic review of the literature. Acta Radiologica. 2019;60:1181-9.
Mai J, Abubrig A, Lehmann T, et al. T2 mapping in prostate cancer. Invest Radiol. 2019;54:146-52.
Klingebiel M, Schimmoller L, Weiland E, et al. Value of T2 mapping MRI for prostate cancer detection and classification. J Magn Reson Img. 2022;54:413-22.
Acknowledgements
We would like to acknowledge Kathy J. Brown and Corey C. Woxland, R.T., for assistance with the human studies and Nicholas B. Larson, Ph.D., for consultation on statistical evaluation.
Funding
National Institutes of Health RR018898; National Institutes of Health R01 EB031790; Mayo Discovery-Translation Program; Mayo Imaging Biomarker Program; General Electric Healthcare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S. J. Riederer and E. A. Borisch. The first draft of the manuscript was written by S. J. Riederer, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SJR and EAB are inventors of a US patent related to the technology discussed. ATF, AK, and NT declare no relevant financial or non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file6 (MP4 39946 kb)
Supplementary file7 (MP4 42759 kb)
Supplementary file8 (MP4 30339 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riederer, S.J., Borisch, E.A., Froemming, A.T. et al. Comparison of model-based versus deep learning-based image reconstruction for thin-slice T2-weighted spin-echo prostate MRI. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04256-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04256-1