Abstract
Purpose
Prostate-specific membrane antigen (PSMA) is a promising target for diagnosis and radioligand therapy (RLT) of prostate cancer. Two novel PSMA-targeting radionuclide therapy agents, [177Lu]Lu-P17-087, and its albumin binder modified derivative, [177Lu]Lu-P17-088, were evaluated in metastatic castration-resistant prostate cancer (mCRPC) patients. The primary endpoint was dosimetry evaluation, the second endpoint was radiation toxicity assessment (CTCAE 5.0) and PSA response (PCWG3).
Methods
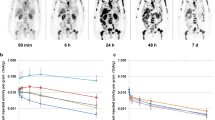
Patients with PSMA-positive tumors were enrolled after [68Ga]Ga-PSMA-11 PET/CT scan. Five mCRPC patients received [177Lu]Lu-P17-087 and four other patients received [177Lu]Lu-P17-088 (1.2 GBq/patient). Multiple whole body planar scintigraphy was performed at 1.5, 4, 24, 48, 72, 120 and 168 h after injection and one SPECT/CT imaging was performed at 24 h post-injection for each patient. Dosimetry evaluation was compared in both patient groups.
Results
Patients showed no major clinical side-effects under this low dose treatment. As expected [177Lu]Lu-P17-088 with longer blood circulation (due to its albumin binding) exhibited higher effective doses than [177Lu]Lu-P17-087 (0.151 ± 0.036 vs. 0.056 ± 0.019 mGy/MBq, P = 0.001). Similarly, red marrow received 0.119 ± 0.068 and 0.048 ± 0.020 mGy/MBq, while kidney doses were 0.119 ± 0.068 and 0.046 ± 0.022 mGy/MBq, respectively. [177Lu]Lu-P17-087 demonstrated excellent tumor uptake and faster kinetics; while [177Lu]Lu-P17-088 displayed a slower washout and higher average dose (7.75 ± 4.18 vs. 4.72 ± 2.29 mGy/MBq, P = 0.018). After administration of [177Lu]Lu-P17-087 and [177Lu]Lu-P17-088, 3/5 and 3/4 patients showed reducing PSA values, respectively.
Conclusion
[177Lu]Lu-P17-088 and [177Lu]Lu-P17-087 displayed different pharmacokinetics but excellent PSMA-targeting dose delivery in mCRPC patients. These two agents are promising RLT agents for personalized treatment of mCRPC. Further studies with increased dose and frequency of RLT are warranted to evaluate the potential therapeutic efficacy.
Trial registration
177Lu-P17-087/177Lu-P17-088 in Patients with Metastatic Castration-resistant Prostate Cancer (NCT05603559, Registered at 25 October, 2022).
URL of registry







Similar content being viewed by others
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Weber WA, Barthel H, Bengel F, Eiber M, Herrmann K, Schäfers M, What. Is Theranostics? J Nucl Med. 2023;64:669–70. https://doi.org/10.2967/jnumed.123.265670.
Jang A, Kendi AT, Sartor O. Status of PSMA-targeted radioligand therapy in prostate cancer: current data and future trials. Ther Adv Med Oncol. 2023;15:1–12.
Sartor O, Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385. https://doi.org/10.1056/NEJMoa2107322.
Hennrich U, Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): the first FDA-Approved Radiotherapeutical for treatment of prostate Cancer. Pharmaceuticals. 2022;15:1292.
Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2023:1–16.
Zang J, Fan X, Wang H, Liu Q, Wang J, Li H, et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:148–58. https://doi.org/10.1007/s00259-018-4096-y.
Zang J, Wang G, Zhao T, Liu H, Lin X, Yang Y, et al. A phase 1 trial to determine the maximum tolerated dose and patient-specific dosimetry of [177Lu]Lu-LNC1003 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2023;51:871–82. https://doi.org/10.1007/s00259-023-06470-3.
Kramer V, Fernández R, Lehnert W, Jiménez-Franco LD, Soza-Ried C, Eppard E, et al. Biodistribution and dosimetry of a single dose of albumin-binding ligand [177Lu]Lu-PSMA-ALB-56 in patients with mCRPC. Eur J Nucl Med Mol Imaging. 2021;48:893–903. https://doi.org/10.1007/s00259-020-05022-3.
Banerjee S, Pillai MR, Knapp FF. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem Rev. 2015;115:2934–74. https://doi.org/10.1021/cr500171e.
Alati S, Singh R, Pomper MG, Rowe SP, Banerjee SR. Preclinical Development in Radiopharmaceutical Therapy for prostate Cancer. Semin Nucl Med. 2023;0:1–12. https://doi.org/10.1053/j.semnuclmed.2023.06.007.
Debnath S, Zhou N, McLaughlin M, Rice S, Pillai AK, Hao G, et al. PSMA-Targeting Imaging and Theranostic agents-current status and future perspective. Int J Mol Sci. 2022;23:1158–77. https://doi.org/10.3390/ijms23031158.
Jackson P, Hofman M, McIntosh L, Buteau JP, Ravi Kumar A. Radiation Dosimetry in 177Lu-PSMA-617 therapy. Semin Nucl Med. 2022;52:243–54. https://doi.org/10.1053/j.semnuclmed.2021.11.003.
Ljungberg M, Celler A, Konijnenberg MW, Eckerman KF, Dewaraja YK, Sjögreen-Gleisner K. MIRD Pamphlet 26: Joint EANM/MIRD guidelines for quantitative 177Lu SPECT Applied for Dosimetry of Radiopharmaceutical Therapy. J Nucl Med. 2016;57:151–62. https://doi.org/10.2967/jnumed.115.159012.
Dale RG. Dose-rate effects in targeted radiotherapy. Phys Med Biol. 1996;41:1871–84. https://doi.org/10.1088/0031-9155/41/10/001.
Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DAL. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol. 2001;13:71–81. https://doi.org/10.1053/clon.2001.9221.
Zha Z, Choi SR, Li L, Zhao R, Ploessl K, Yao X, et al. New PSMA-Targeting ligands: Transformation from diagnosis (Ga-68) to Radionuclide Therapy (Lu-177). J Med Chem. 2022;65:13001–12. https://doi.org/10.1021/acs.jmedchem.2c00852.
Jeitner TM, Babich JW, Kelly JM. Advances in PSMA theranostics. Transl Oncol. 2022;22:101450. https://doi.org/10.1016/j.tranon.2022.101450.
Sjögreen Gleisner K, Chouin N, Gabina PM, Cicone F, Gnesin S, Stokke C. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur J Nucl Med Mol Imaging. 2022;49. https://doi.org/10.1007/s00259-022-05727-7.
Evangelista L, Maurer T, van der Poel H, Alongi F, Kunikowska J, Laudicella R, et al. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the staging of primary and recurrent prostate Cancer. A systematic review of the literature. Eur Urol Oncol. 2022;5:273–82. https://doi.org/10.1016/j.euo.2022.03.004.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. https://doi.org/10.1016/s0140-6736(21)00237-3.
Hippeläinen ET, Tenhunen MJ, Mäenpää HO, Heikkonen JJ, Sohlberg AO. Dosimetry software Hermes Internal Radiation Dosimetry: from quantitative image reconstruction to voxel-level absorbed dose distribution. Nucl Med Commun. 2017;38:357–65. https://doi.org/10.1097/MNM.0000000000000662.
Schuchardt C, Zhang J, Kulkarni HR, Chen X, Müller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J Nucl Med. 2022;63:1199–207.
Feuerecker B, Chantadisai M, Allmann A, Tauber R, Allmann J, Steinhelfer L. Pretherapeutic comparative dosimetry of 177Lu-rhPSMA-7.3 and 177Lu-PSMA I&T in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2022;63:833–9. https://doi.org/10.2967/jnumed.121.262671.
Zang J, Liu Q, Sui H, Wang R, Jacobson O, Fan X, et al. 177Lu-EB-PSMA Radioligand Therapy with escalating doses in patients with metastatic castration-resistant prostate Cancer. J Nucl Med. 2020;61:1772–8. https://doi.org/10.2967/jnumed.120.242263.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34. https://doi.org/10.1200/JCO.2015.64.2702.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. https://doi.org/10.1016/s1470-2045(18)30198-0.
Zha Z, Ploessl K, Choi SR, Wu Z, Zhu L, Kung HF. Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor. Nucl Med Biol. 2018;59:36–47. https://doi.org/10.1016/j.nucmedbio.2017.12.007.
Green MA, Hutchins GD, Bahler CD, Tann M, Mathias CJ, Territo W, et al. [68Ga]Ga-P16-093 as a PSMA-Targeted PET Radiopharmaceutical for detection of Cancer: initial evaluation and comparison with [68Ga]Ga-PSMA-11 in prostate Cancer patients presenting with biochemical recurrence. Mol Imaging Biol. 2020;22:752–63. https://doi.org/10.1007/s11307-019-01421-7.
Lee H, Scheuermann JS, Young AJ, Doot RK, Daube-Witherspoon ME, Schubert EK, et al. Preliminary evaluation of 68Ga-P16-093, a PET Radiotracer Targeting prostate-specific membrane Antigen in prostate Cancer. Mol Imaging Biol. 2022;26:1–11.
Wang G, Hong H, Zang J, Liu Q, Jiang Y, Fan X, et al. Head-to-head comparison of [68Ga]Ga-P16-093 and [68Ga]Ga-PSMA-617 in dynamic PET/CT evaluation of the same group of recurrent prostate cancer patients. Eur J Nucl Med Mol Imaging. 2022;49:1052–62. https://doi.org/10.1007/s00259-021-05539-1.
Dumelin CE, Scheuermann J, Melkko S, Neri D. Selection of streptavidin binders from a DNA-encoded chemical library. Bioconjug Chem. 2006;17:366–70.
Dumelin CE, Trüssel S, Buller F, Trachsel E, Bootz F, Zhang Y, et al. A portable albumin Binder from a DNA-Encoded Chemical Library. Angew Chem Int Ed. 2008;47:3196–201. https://doi.org/10.1002/anie.200704936.
Kelly JM, Amor-Coarasa A, Ponnala S, Nikolopoulou A, Williams C Jr., DiMagno SG, et al. Albumin-binding PSMA ligands: implications for expanding the therapeutic window. J Nucl Med. 2019;60:656–63. https://doi.org/10.2967/jnumed.118.221150.
Umbricht CA, Benesova M, Schibli R, Muller C, Kelly J, Amor-Coarasa A, et al. Preclinical Development of Novel PSMA-Targeting Radioligands: modulation of albumin-binding. Mol Pharm. 2018;15:2297–306. https://doi.org/10.1021/acs.molpharmaceut.8b00152.
Sidaway P. 177Lu-PSMA-PET extends survival. Nat Rev Clin Oncol. 2021;18:510. https://doi.org/10.1038/s41571-021-00543-8.
Benesova M, Umbricht CA, Schibli R, Muller C, Albumin-Binding PSMA, Ligands. Optimization of the tissue distribution Profile. Mol Pharm. 2018;15:934–46. https://doi.org/10.1021/acs.molpharmaceut.7b00877.
Kuo HT, Merkens H, Zhang Z, Uribe CF, Lau J, Zhang C, et al. Enhancing treatment efficacy of 177Lu-PSMA-617 with the conjugation of an albumin-binding motif: Preclinical Dosimetry and Endoradiotherapy studies. Mol Pharm. 2018;15:5183–91. https://doi.org/10.1021/acs.molpharmaceut.8b00720.
Kuo HT, Lin KS, Zhang Z, Uribe CF, Merkens H, Zhang C, et al. 177Lu-Labeled Albumin-Binder-Conjugated PSMA-Targeting agents with extremely high Tumor Uptake and enhanced tumor-to-kidney absorbed dose ratio. J Nucl Med. 2021;62:521–7. https://doi.org/10.2967/jnumed.120.250738.
Ha S, Park C, Boo SH, Yoo IR, Moon HW, Chi DY, et al. Dosimetric Analysis of a phase I study of PSMA-Targeting Radiopharmaceutical Therapy with [177Lu]ludotadipep in patients with metastatic castration-resistant prostate Cancer. Korean J Radiol. 2024;25:179–88.
Kramer V, Fernández R, Lehnert W, Jiménez-Franco LD, Soza-Ried C, Eppard E, et al. Biodistribution and dosimetry of a single dose of albumin-binding ligand [177Lu]Lu-PSMA-ALB-56 in patients with mCRPC. Eur J Nucl Med Mol Imaging. 2021;48:893–903.
Borgna F, Deberle LM, Cohrs S, Schibli R, Müller C. Combined application of albumin-binding [177Lu]Lu-PSMA-ALB-56 and fast-cleared psma inhibitors: optimization of the Pharmacokinetics. Mol Pharm. 2020;17:2044–53.
Derlin T, Riethdorf S, Schumacher U, Lafos M, Peine S, Coith C, et al. PSMA-heterogeneity in metastatic castration‐resistant prostate cancer: circulating tumor cells, metastatic tumor burden, and response to targeted radioligand therapy. Prostate. 2023;1–13. https://doi.org/10.1002/pros.24549.
Acknowledgements
Authors thank Drs. David Mankoff, Daniel Pryma (University of Pennsylvania) and Dr. William Eckelman (Five Eleven Pharma Inc.) for their valuable suggestions to this project. In loving memory of Tongyuan Cui (1995.8.5–2023.8.20) from Department of Engineering Physics, Tsinghua University, who encouraged and guided me (Linlin Li) towards making my career choice in radiopharmaceutical and nuclear medicine research.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-004), the Fundamental Research Funds for the Central Universities (3332022110), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2021-I2M-1-016, 2022-I2M-C&T-A-008, 2022-I2M-2-002) and the National Natural Science Foundation of China (82272046, 22176016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Institute Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (no. ZS-3567), and registered at clinicaltrials.gov (NCT05603559).
Conflict of interest
David Alexoff, Karl Ploessl and Hank F. Kung are employees of Five Eleven Pharma Inc. which holds the patent rights for [177Lu]Lu-P17-087 and [177Lu]Lu-P17-088 and related technology. Dr. Hank F. Kung is also the Founder and Chairman of the board at Five Eleven Pharma Inc. Other authors declare no other conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Wang, J., Wang, G. et al. Comparison of novel PSMA-targeting [177Lu]Lu-P17-087 with its albumin binding derivative [177Lu]Lu-P17-088 in metastatic castration-resistant prostate cancer patients: a first-in-human study. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06721-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06721-x