Abstract
Purpose
To improve the diagnostic accuracy of initial detection in patients with suspected primary prostate cancer (PCa).
Methods
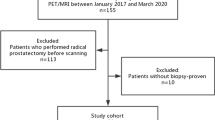
Eighty-four patients who underwent Gallium-68–labeled prostate-specific membrane antigen ([68Ga]Ga-PSMA-11) total-body positron emission tomography/computed tomography (PET/CT) imaging before treatment in our department were enrolled. The maximum standard uptake value (SUVmax) of the prostate (SUVmax-PSMA), liver (SUVmax-PSMA-L), and mediastinal blood pool (SUVmax-PSMA-M) was measured using [68Ga]Ga-PSMA-11 total-body PET/CT imaging. The [68Ga]Ga-PSMA-11 derived metabolic tumor volume (MTV), the total lesion (TLP), and the cross-sectional areas of focal concentration in the prostate (CAP) were also determined. Besides, the prostate-specific antigen (PSA) levels and the above imaging characteristics were analyzed using receiver operating characteristic curves to identify the cutoff value to improve the diagnostic accuracy of suspected PCa. Finally, a multivariate regression analysis was conducted to discover the independent predictor to improve the diagnostic accuracy on [68Ga]Ga-PSMA-11 total-body imaging.
Results
There was no significant difference between the PCa and Non-PCa groups in age, height, weight, injected dose, except for the PSA levels, the SUVmax-PSMA, TLP, MTV, and CAP. Besides, the SUVmax-PSMA-T/L and SUVmax-PSMA-T/M derived from SUVmax-PSMA were both significantly different. In addition, the areas under the curve of PSA levels, SUVmax-PSMA, SUVmax-PSMA-T/L, SUVmax-PSMA-T/M, TLP, MTV, and CAP to predict PCa on [68Ga]Ga-PSMA-11 imaging were 0.620 (95% confidence interval (CI) 0.485–0.755), 0.864 (95% CI 0.757–0.972), 0.819 (95% CI 0.704–0.935), 0.876 (95% CI 0.771–0.980), 0.845 (95% CI 0.741–0.949), 0.820 (95% CI 0.702–0.938), 0.627 (95% CI 0.499–0.754), respectively. However, a multivariate regression analysis showed that SUVmax-PSMA was an independent predictor, with a cutoff value of 11.5 and an odds ratio of 1.221.
Conclusion
The SUVmax-PSMA with a cutoff value of 11.5 was an independent predictor to improve the diagnostic accuracy of PCa on [68Ga]Ga-PSMA-11 total-body imaging.




Similar content being viewed by others
Data availability
The dataset used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Prcic A, Begic E, Hiros M. Usefulness of total PSA value in prostate diseases diagnosis. Acta Inform Med. 2016;24(3):156–61.
Lomas DJ, Ahmed HU. All change in the prostate cancer diagnostic pathway. Nat Rev Clin Oncol. 2020;17(6):372–81.
Ghai S, Haider MA. Multiparametric-MRI in diagnosis of prostate cancer. Indian J Urol. 2015;31(3):194–201.
Zhen L, Liu X, Yegang C, Yongjiao Y, Yawei X, Jiaqi K, et al. Accuracy of multiparametric magnetic resonance imaging for diagnosing prostate cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):1244.
Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology. 2016;280(3):793–804.
Richenberg J, Logager V, Panebianco V, Rouviere O, Villeirs G, Schoots IG. The primacy of multiparametric MRI in men with suspected prostate cancer. Eur Radiol. 2019;29(12):6940–52.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74.
Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41(5):887–97.
Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70(5):829–36.
Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44(6):941–9.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77(4):403–17.
Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of (68)Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J Nucl Med. 2020;61(12):1793–9.
O’Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. 1998;1443(1–2):113–27.
Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using multiple tumour tissue microarray technique. Histopathology. 2007;50(4):472–83.
Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–72.
Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76(4):469–78.
Pizzuto DA, Muller J, Muhlematter U, Rupp NJ, Topfer A, Mortezavi A, et al. The central zone has increased (68)Ga-PSMA-11 uptake: “Mickey Mouse ears” can be hot on (68)Ga-PSMA-11 PET. Eur J Nucl Med Mol Imaging. 2018;45(8):1335–43.
Ferraro DA, Rupp NJ, Donati OF, Messerli M, Eberli D, Burger IA. 68Ga-PSMA-11 PET/MR can be false positive in normal prostatic tissue. Clin Nucl Med. 2019;44(4):e291–3.
Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59(1):3–12.
Zhang X, Badawi RD, Cherry SR, Qi J. Theoretical study of the benefit of long axial field-of-view PET on region of interest quantification. Phys Med Biol. 2018;63(13): 135010.
Cherry SR, Badawi RD, Karp JS, Moses WW, Price P, Jones T. Total-body imaging: transforming the role of positron emission tomography. Sci Transl Med. 2017;9(381):eaaf6169.
Surti S, Karp JS. Impact of detector design on imaging performance of a long axial field-of-view, whole-body PET scanner. Phys Med Biol. 2015;60(13):5343–58.
Tan H, Sui X, Yin H, Yu H, Gu Y, Chen S, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(6):1966–75.
Liu G, Hu P, Yu H, Tan H, Zhang Y, Yin H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48(8):2373–83.
Lv J, Yin H, Yu H, Liu G, Shi H. The feasibility of ultralow-activity (18)F-FDG dynamic PET imaging in lung adenocarcinoma patients through total-body PET/CT scanner. Ann Nucl Med. 2022;36(10):887–96.
Hu Y, Liu G, Yu H, Wang Y, Li C, Tan H, et al. Feasibility of acquisitions using total-body PET/CT with an ultra-low (18)F-FDG activity. J Nucl Med. 2022;63(6):959–65.
Liu C, Liu T, Zhang Z, Zhang N, Du P, Yang Y, et al. (68)Ga-PSMA PET/CT combined with PET/ultrasound-guided prostate biopsy can diagnose clinically significant prostate cancer in men with previous negative biopsy results. J Nucl Med. 2020;61(9):1314–9.
van Rossum PSN, Fried DV, Zhang L, Hofstetter WL, Ho L, Meijer GJ, et al. The value of (18)F-FDG PET before and after induction chemotherapy for the early prediction of a poor pathologic response to subsequent preoperative chemoradiotherapy in oesophageal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2017;44(1):71–80.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Moller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43(7):1209–19.
Husby JA, Reitan BC, Biermann M, Trovik J, Bjorge L, Magnussen IJ, et al. Metabolic tumor volume on 18F-FDG PET/CT improves preoperative identification of high-risk endometrial carcinoma patients. J Nucl Med. 2015;56(8):1191–8.
Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic accuracy of (68)Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2021;216(3):599–607.
Shetty D, Patel D, Le K, Bui C, Mansberg R. Pitfalls in Gallium-68 PSMA PET/CT interpretation—a pictorial review. Tomography. 2018;4(4):182–93.
Jain H, Sood R, Faridi MS, Goel H, Sharma U. Role of 68Ga-PSMA-PET/CT for the detection of primary prostate cancer prior to biopsy: a prospective study. Cent European J Urol. 2021;74(3):315–20.
Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189(2):323–8.
de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202(2):343–51.
Kuru TH, Fütterer JJ, Schiffmann J, Porres D, Salomon G, Rastinehad AR. Transrectal ultrasound (US), contrast-enhanced US, real-time elastography, HistoScanning, magnetic resonance imaging (MRI), and MRI-US fusion biopsy in the diagnosis of prostate cancer. Eur Urol Focus. 2015;1(2):117–26.
Amin A, Blazevski A, Thompson J, Scheltema MJ, Hofman MS, Murphy D, et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic resonance imaging in the diagnostic setting for men being investigated for prostate cancer. BJU Int. 2020;125(4):515–24.
Emmett L, Papa N, Buteau J, Ho B, Liu V, Roberts M, et al. The PRIMARY score: using intraprostatic (68)Ga-PSMA PET/CT patterns to optimize prostate cancer diagnosis. J Nucl Med. 2022;63(11):1644–50.
Funding
This study was funded by the Shanghai Municipal Key Clinical Specialty Project (grant number: SHSLCZDZK03401), the Major Science and Technology Projects for Major New Drug Creation (grant number: 2019ZX09302001), the Shanghai Science and Technology Committee program (grant number: 20DZ2201800), the Three-year Action Plan of Clinical Skills and Innovation of Shanghai Hospital Development Center (grant number: SHDC2020CR3079B), and the Shanghai Sailing Program (19YF1408200).
Author information
Authors and Affiliations
Contributions
JL and HS were responsible for the design of the study. JL, HY, and HY were involved in data acquisition. JL, HY, and YS participated in data analysis. JL and HS drafted the manuscript and improved the performance of the research.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional review board of Zhongshan Hospital, Fudan University (IRB number: B2020-266R). Written informed consent was obtained from all participants.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, J., Yu, H., Yin, H. et al. A single-center, multi-factor, retrospective study to improve the diagnostic accuracy of primary prostate cancer using [68Ga]Ga-PSMA-11 total-body PET/CT imaging. Eur J Nucl Med Mol Imaging 51, 919–927 (2024). https://doi.org/10.1007/s00259-023-06464-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06464-1