Abstract
Purpose
Myocardial ischemia–reperfusion (I/R) injury is associated with systemic oxidative stress, cardiac mitochondrial homeostasis, and cardiomyocyte apoptosis. Metformin has been recognized to attenuate cardiomyocyte apoptosis. However, the longitudinal effects and pathomechanism of metformin on the regulation of myocardial mitohormesis following I/R treatment remain unclear. This study aimed to investigate the longitudinal effects and mechanism of metformin in regulating cardiac mitochondrial homeostasis by serial imaging with the 18-kDa translocator protein (TSPO)–targeted positron emission tomography (PET) tracer 18F-FDPA.
Methods
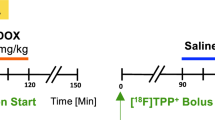
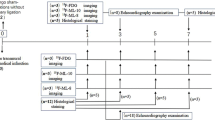
Myocardial I/R injury was established in Sprague–Dawley rats, which were treated with or without metformin (150 mg/kg per day). Serial gated 18F-FDG and 18F-FDPA PET imaging were performed at 1, 4, and 8 weeks after surgery, followed by analysis of ventricular remodelling and cardiac mitochondrial homeostasis. The correlation between Hsp60 and 18F-FDPA uptake was analyzed. After PET imaging, the activity of antioxidant enzymes, immunostaining, and western blot analysis were performed to analyze the spatio-temporal effects and pathomechanism of metformin for cardiac protection after myocardial I/R injury.
Results
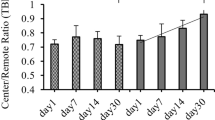
Oxidative stress and apoptosis increased 1 week after myocardial I/R injury (before significant progression of ventricular remodelling). TSPO expression was correlated with Hsp60 expression and was co-localized with inflammatory CD68+ macrophages in the infarct area, and TSPO uptake was associated with an upregulation of AMPK-p/AMPK and a downregulation of Bcl-2/Bax. However, these effects were reversed with metformin treatment. Eight weeks after myocardial I/R injury (representing the advanced stage of heart failure), 18F-FDPA uptake in myocardial cells in the distal non-infarct area increased without CD68+ expression, whereas the activity decreased with metformin treatment.
Conclusion
Taken together, these results show that a prolonged metformin treatment has pleiotropic protective effects against myocardial I/R injury associated with a regional and temporal dynamic balance between mitochondrial homeostasis and cardiac outcome, which were assessed by TSPO-targeted imaging during cardiac remodelling.








Similar content being viewed by others
References
Higuchi T, Fukushima K, Xia J, Mathews WB, Lautamaki R, Bravo PE, et al. Radionuclide imaging of angiotensin II type 1 receptor upregulation after myocardial ischemia-reperfusion injury. J Nucl Med. 2010;51:1956–61. https://doi.org/10.2967/jnumed.110.079855.
Kloner RA. Stunned and hibernating myocardium: where are we nearly 4 decades later? J Am Heart Assoc. 2020;9:e015502. https://doi.org/10.1161/JAHA.119.015502.
Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–70. https://doi.org/10.1038/s41569-018-0044-6.
Meng Y, Tian M, Yin S, Lai S, Zhou Y, Chen J, et al. Downregulation of TSPO expression inhibits oxidative stress and maintains mitochondrial homeostasis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed Pharmacother. 2020;121:109588. https://doi.org/10.1016/j.biopha.2019.109588.
Lu LQ, Tian J, Luo XJ, Peng J. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci. 2021;78:63–78. https://doi.org/10.1007/s00018-020-03587-8.
Salameh A, Dhein S, Mewes M, Sigusch S, Kiefer P, Vollroth M, et al. Anti-oxidative or anti-inflammatory additives reduce ischemia/reperfusions injury in an animal model of cardiopulmonary bypass. Saudi J Biol Sci. 2020;27:18–29. https://doi.org/10.1016/j.sjbs.2019.04.003.
Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–9. https://doi.org/10.1016/j.febslet.2007.11.018.
Morrison A, Li J. PPAR-gamma and AMPK–advantageous targets for myocardial ischemia/reperfusion therapy. Biochem Pharmacol. 2011;82:195–200. https://doi.org/10.1016/j.bcp.2011.04.004.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35. https://doi.org/10.1038/nrm.2017.95.
Chen X, Li X, Zhang W, He J, Xu B, Lei B, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kappaB pathway. Metabolism. 2018;83:256–70. https://doi.org/10.1016/j.metabol.2018.03.004.
Lau S, Patnaik N, Sayen MR, Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–94. https://doi.org/10.1161/01.cir.96.7.2287.
Zhou C, Sun H, Zheng C, Gao J, Fu Q, Hu N, et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018;9:161. https://doi.org/10.1038/s41419-017-0196-z.
Taliani S, Pugliesi I, Da Settimo F. Structural requirements to obtain highly potent and selective 18 kDa Translocator Protein (TSPO) Ligands. Curr Top Med Chem. 2011;11:860–86. https://doi.org/10.2174/156802611795165142.
Gavish M, Veenman L. Regulation of mitochondrial, cellular, and organismal functions by TSPO. Adv Pharmacol. 2018;82:103–36. https://doi.org/10.1016/bs.apha.2017.09.004.
Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol. 2018;71:263–75. https://doi.org/10.1016/j.jacc.2017.11.024.
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–88. https://doi.org/10.1038/nrd3295.
Mou T, Tian J, Tian Y, Yun M, Li J, Dong W, et al. Automated synthesis and preliminary evaluation of [(18)F]FDPA for cardiac inflammation imaging in rats after myocardial infarction. Sci Rep. 2020;10:18685. https://doi.org/10.1038/s41598-020-75705-2.
Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67. https://doi.org/10.1161/01.cir.97.22.2259.
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. 2018;113:39. https://doi.org/10.1007/s00395-018-0696-8.
Kurhaluk N, Bojkova B, Radkowski M, Zaitseva OV, Kyriienko S, Demkow U, et al. Melatonin and metformin diminish oxidative stress in heart tissue in a rat model of high fat diet and mammary carcinogenesis. Adv Exp Med Biol. 2018;1047:7–19. https://doi.org/10.1007/5584_2017_128.
Karam HM, Radwan RR. Metformin modulates cardiac endothelial dysfunction, oxidative stress and inflammation in irradiated rats: A new perspective of an antidiabetic drug. Clin Exp Pharmacol Physiol. 2019;46:1124–32. https://doi.org/10.1111/1440-1681.13148.
Zhang X, Liu XJ, Hu S, Schindler TH, Tian Y, He ZX, et al. Long-term survival of patients with viable and nonviable aneurysms assessed by 99mTc-MIBI SPECT and 18F-FDG PET: a comparative study of medical and surgical treatment. J Nucl Med. 2008;49:1288–98. https://doi.org/10.2967/jnumed.107.046730.
Yun M, Nie B, Wen W, Zhu Z, Liu H, Nie S, et al. Assessment of cerebral glucose metabolism in patients with heart failure by (18)F-FDG PET/CT imaging. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02258-2.
Werner RA, Maya Y, Rischpler C, Javadi MS, Fukushima K, Lapa C, et al. Sympathetic nerve damage and restoration after ischemia-reperfusion injury as assessed by (11)C-hydroxyephedrine. Eur J Nucl Med Mol Imaging. 2016;43:312–8. https://doi.org/10.1007/s00259-015-3171-x.
Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, et al. Single Photon Emission Computed Tomography (SPECT) Myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25:1784–846. https://doi.org/10.1007/s12350-018-1283-y.
Autio A, Uotila S, Kiugel M, Kyto V, Liljenback H, Kudomi N, et al. (68)Ga-DOTA chelate, a novel imaging agent for assessment of myocardial perfusion and infarction detection in a rodent model. J Nucl Cardiol. 2020;27:891–8. https://doi.org/10.1007/s12350-019-01752-6.
Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Targeting amino acid metabolism for molecular imaging of inflammation early after myocardial infarction. Theranostics. 2016;6:1768–79. https://doi.org/10.7150/thno.15929.
Wang Y, Yang Z, Zheng G, Yu L, Yin Y, Mu N, et al. Metformin promotes autophagy in ischemia/reperfusion myocardium via cytoplasmic AMPKalpha1 and nuclear AMPKalpha2 pathways. Life Sci. 2019;225:64–71. https://doi.org/10.1016/j.lfs.2019.04.002.
Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. https://doi.org/10.1016/j.freeradbiomed.2018.01.024.
Knowlton AA, Gupta S. HSP60, Bax, and cardiac apoptosis. Cardiovasc Toxicol. 2003;3:263–8. https://doi.org/10.1385/ct:3:3:263.
Wang X, Yang L, Kang L, Li J, Yang L, Zhang J, et al. Metformin attenuates myocardial ischemia-reperfusion injury via up-regulation of antioxidant enzymes. PLoS ONE. 2017;12:e0182777. https://doi.org/10.1371/journal.pone.0182777.
Wu S, Zou MH. AMPK, Mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21(14):4987. https://doi.org/10.3390/ijms21144987
Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–9. https://doi.org/10.1016/j.tem.2015.05.010.
Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–82. https://doi.org/10.1038/nature06504.
de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. https://doi.org/10.1155/2013/206039.
Elkamhawy A, Park JE, Hassan AHE, Pae AN, Lee J, Park BG, et al. Design, synthesis, biological evaluation and molecular modelling of 2-(2-aryloxyphenyl)-1,4-dihydroisoquinolin-3(2H)-ones: a novel class of TSPO ligands modulating amyloid-beta-induced mPTP opening. Eur J Pharm Sci. 2017;104:366–81. https://doi.org/10.1016/j.ejps.2017.04.015.
Farahmand P, Lai TY, Weisel RD, Fazel S, Yau T, Menasche P, et al. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation. 2008;118:S130–7. https://doi.org/10.1161/CIRCULATIONAHA.107.757617.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81871377, 81571717, 82171994) and Beijing Municipal Administration of Hospitals (ZYLX202110) .
Author information
Authors and Affiliations
Contributions
Jing Tian and Xiaoli Zhang designed the study. Tiantian Mou carried out the radiolabelling experiments. Yi Tian, Jing Tian, and Yaqi Zheng performed the animal experiments. Jing Tian, Xiang Li, and Mingkai Yun interpreted and analysed the scans. Jing Tian analyzed and interpreted the data. Jing Tian wrote the first draft of manuscript, which was revised by Marcus Hacker, Xiaoli Zhang, and Xiang Li. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal experiments were performed according to Beijing’s laboratory animal management regulations and were approved by the Animal Care Committee of Capital Medical University (Ethical approval number: AEEI-2019–167).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, J., Zheng, Y., Mou, T. et al. Metformin confers longitudinal cardiac protection by preserving mitochondrial homeostasis following myocardial ischemia/reperfusion injury. Eur J Nucl Med Mol Imaging 50, 825–838 (2023). https://doi.org/10.1007/s00259-022-06008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06008-z