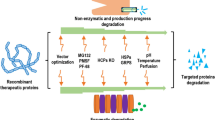
Abstract
Mammalian cell lines are frequently used as the preferred host cells for producing recombinant therapeutic proteins (RTPs) having post-translational modified modifications similar to those observed in proteins produced by human cells. Nowadays, most RTPs approved for marketing are produced in Chinese hamster ovary (CHO) cells. Recombinant therapeutic antibodies (RTAs) are among the most important and promising RTPs for biomedical applications. A major limitation associated with the use of RTAs is their aggregation, which can be caused by a variety of factors; this results in a reduction of quality. RTA aggregations are especially concerning as they can trigger human immune responses in humans and may be fatal. Therefore, the mechanisms underlying RTA aggregation and measures for avoiding aggregation are interesting topics in RTAs research. In this review, we discuss recent progress in the field of RTAs aggregation, with a focus on factors that cause aggregation during RTA production and the development of strategies for overcoming RTA aggregation.
Key points
• The recombinant antibody aggregation in mammalian cell systems is reviewed.
• Intracellular environment and extracellular parameters influence recombinant antibody aggregation.
• Reducing the aggregations can improve the quality of recombinant antibodies.


Similar content being viewed by others
References
Alam ME, Slaney TR, Wu L, Das TK, Kar S, Barnett GV, Leone A, Tessier PM (2020) Unique impacts of methionine oxidation, tryptophan oxidation, and asparagine deamidation on antibody stability and aggregation. J Pharm Sci 109:656–669. https://doi.org/10.1016/j.xphs.2019.10.051
Barzadd MM, Lundqvist M, Harris C, Malm M, Volk AL, Thalén N, Chotteau V, Grassi L, Smith A, Abadi ML, Lambiase G, Gibson S, Hatton D, Rockberg J (2022) Autophagy and intracellular product degradation genes identified by systems biology analysis reduce aggregation of bispecific antibody in CHO cells. N Biotechnol 68:68–76. https://doi.org/10.1016/j.nbt.2022.01.010
Bayat H, Hossienzadeh S, Pourmaleki E, Ahani R, Rahimpour A (2018) Evaluation of different vector design strategies for the expression of recombinant monoclonal antibody in CHO cells. Prep Biochem Biotechnol 48:160–164. https://doi.org/10.1080/10826068.2017.1421966
Bickel F, Herold EM, Signes A, Romeijn S, Jiskoot W, Kiefer H (2016) Reversible NaCl-induced aggregation of a monoclonal antibody at low pH: characterization of aggregates and factors affecting aggregation. Eur J Pharm Biopharm 107:310–320. https://doi.org/10.1016/j.ejpb.2016.07.020
Borth N, Mattanovich D, Kunert R, Katinger H (2005) Effect of increased expression of protein disulfide isomerase and heavy chain binding protein on antibody secretion in a recombinant CHO cell line. Biotechnol Prog 21:106–111. https://doi.org/10.1021/bp0498241
Brinkmann U, Kontermann RE (2017) The Making of Bispecific Antibodies. Mabs 9:182–212. https://doi.org/10.1080/19420862.2016.1268307
Buchanan A, Clementel V, Woods R, Harn N, Bowen MA, Mo W, Popovic B, Bishop SM, Dall’Acqua W, Minter R, Jermutus L, Bedian V (2013) Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression. Mabs 5:255–262. https://doi.org/10.4161/mabs.23392
Chennamsetty N, Helk B, Voynov V, Kayser V, Trout BL (2009) Aggregation-prone motifs in human immunoglobulin G. J Mol Biol 391:404–413. https://doi.org/10.1016/j.jmb.2009.06.028
Cromwell ME, Hilario E, Jacobson F (2006) Protein Aggregation and Bioprocessing. Aaps j 8:E572-579. https://doi.org/10.1208/aapsj080366
Dahodwala H, Lee KH (2019) The fickle CHO: a review of the causes, implications, and potential alleviation of the CHO cell line instability problem. Curr Opin Biotechnol 60:128–137. https://doi.org/10.1016/j.copbio.2019.01.011
De Nardis C, Hendriks LJA, Poirier E, Arvinte T, Gros P, Bakker ABH, de Kruif J (2017) A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G(1). J Biol Chem 292:14706–14717. https://doi.org/10.1074/jbc.M117.793497
Doronina VA, Wu C, de Felipe P, Sachs MS, Ryan MD, Brown JD (2008) Site-specific release of nascent chains from ribosomes at a sense codon. Mol Cell Biol 28:4227–4239. https://doi.org/10.1128/mcb.00421-08
Eszterhas SK, Bouhassira EE, Martin DI, Fiering S (2002) Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol Cell Biol 22:469–479. https://doi.org/10.1128/mcb.22.2.469-479.2002
Freilich R, Arhar T, Abrams JL, Gestwicki JE (2018) Protein-protein interactions in the molecular chaperone network. Acc Chem Res 51:940–949. https://doi.org/10.1021/acs.accounts.8b00036
Gangwar N, Mishra R, Budholiya N, Rathore AS (2021) Effect of vitamins and metalions on productivity and charge heterogeneity of IgG1 expressed in CHO cells. Biotechnol J 16:e2000464. https://doi.org/10.1002/biot.202000464
Gomez N, Subramanian J, Ouyang J, Nguyen MD, Hutchinson M, Sharma VK, Lin AA, Yuk IH (2012) Culture temperature modulates aggregation of recombinant antibody in cho cells. Biotechnol Bioeng 109:125–136. https://doi.org/10.1002/bit.23288
Gomez N, Wieczorek A, Lu F, Bruno R, Diaz L, Agrawal NJ, Daris K (2018) Culture temperature modulates half antibody and aggregate formation in a Chinese hamster ovary cell line expressing a bispecific antibody. Biotechnol Bioeng 115:2930–2940. https://doi.org/10.1002/bit.26803
Gomez N, Barkhordarian H, Lull J, Huh J, GhattyVenkataKrishna P, Zhang X (2019) Perfusion CHO cell culture applied to lower aggregation and increase volumetric productivity for a bispecific recombinant protein. J Biotechnol 304:70–77. https://doi.org/10.1016/j.jbiotec.2019.08.001
Gonzlez R, Andrews BA, Asenjo JA (2002) Kinetic model of BiP- and PDI-mediated protein folding and assembly. J Theor Biol 214:529–537. https://doi.org/10.1006/jtbi.2001.2478
Handlogten MW, Lee-O’Brien A, Roy G, Levitskaya SV, Venkat R, Singh S, Ahuja S (2018) Intracellular response to process optimization and impact on productivity and product aggregates for a high-titer CHO cell process. Biotechnol Bioeng 115:126–138. https://doi.org/10.1002/bit.26460
Hari SB, Lau H, Razinkov VI, Chen S, Latypov RF (2010) Acid-induced aggregation of human monoclonal IgG1 and IgG2: molecular mechanism and the effect of solution composition. Biochemistry 49:9328–9338. https://doi.org/10.1021/bi100841u
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. https://doi.org/10.1038/nrm3270
Ho SC, Bardor M, Feng H, Mariati TYW, Song Z, Yap MG, Yang Y (2012) IRES-mediated Tricistronic vectors for enhancing generation of high monoclonal antibody expressing CHO cell lines. J Biotechnol 157:130–139. https://doi.org/10.1016/j.jbiotec.2011.09.023
Ho SC, Bardor M, Li B, Lee JJ, Song Z, Tong YW, Goh LT, Yang Y (2013) Comparison of internal ribosome entry site (IRES) and Furin-2A (F2A) for monoclonal antibody expression level and quality in CHO cells. PLoS One 8:e63247. https://doi.org/10.1371/journal.pone.0063247
Ho SC, Wang T, Song Z, Yang Y (2015) IgG aggregation mechanism for CHO cell lines expressing excess heavy chains. Mol Biotechnol 57:625–634. https://doi.org/10.1007/s12033-015-9852-7
Hong JK, Lee SM, Kim KY, Lee GM (2014) Effect of sodium butyrate on the assembly, charge variants, and galactosylation of antibody produced in recombinant Chinese hamster ovary cells. Appl Microbiol Biotechnol 98:5417–5425. https://doi.org/10.1007/s00253-014-5596-8
Huang S, Segués A, Hulsik DL, Zaiss DM, Sijts A, van Duijnhoven SMJ, van Elsas A (2020) A novel efficient bispecific antibody format, combining a conventional antigen-binding fragment with a single domain antibody, avoids potential heavy-light chain mis-pairing. J Immunol Methods 483:112811. https://doi.org/10.1016/j.jim.2020.112811
Imamura H, Honda S (2016) Kinetics of antibody aggregation at neutral pH and ambient temperatures triggered by temporal exposure to acid. J Phys Chem B 120:9581–9589. https://doi.org/10.1021/acs.jpcb.6b05473
Jia YL, Guo X, Lu JT, Wang XY, Qiu LL, Wang TY (2018) CRISPR/Cas9-mediated gene knockout for DNA methyltransferase Dnmt3a in CHO cells displays enhanced transgenic expression and long-term stability. J Cell Mol Med 22:4106–4116. https://doi.org/10.1111/jcmm.13687
Jin W, Xing Z, Song Y, Huang C, Xu X, Ghose S, Li ZJ (2019) Protein aggregation and mitigation strategy in low pH viral inactivation for monoclonal antibody purification. Mabs 11:1479–1491. https://doi.org/10.1080/19420862.2019.1658493
Kaplon H, Muralidharan M, Schneider Z, Reichert JM (2020) Antibodies to watch in 2020. Mabs 12:1703531. https://doi.org/10.1080/19420862.2019.1703531
Kellner K, Solanki A, Amann T, Lao N, Barron N (2018) Targeting miRNAs with CRISPR/Cas9 to improve recombinant protein production of CHO cells. Methods Mol Biol 1850:221–235. https://doi.org/10.1007/978-1-4939-8730-6_15
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355. https://doi.org/10.1146/annurev-biochem-060208-092442
Kim HS, Dunshee DR, Yee A, Tong RK, Kim I, Farahi F, Hongo JA, Ernst JA, Sonoda J, Spiess C (2017) Tethered-variable CL bispecific IgG: an antibody platform for rapid bispecific antibody screening. Protein Eng Des Sel 30:627–637. https://doi.org/10.1093/protein/gzx034
Lee CJ, Seth G, Tsukuda J, Hamilton RW (2009) A clone screening method using mRNA levels to determine specific productivity and product quality for monoclonal antibodies. Biotechnol Bioeng 102:1107–1118. https://doi.org/10.1002/bit.22126
Li W, Prabakaran P, Chen W, Zhu Z, Feng Y, Dimitrov DS (2016) Antibody aggregation: insights from sequence and structure. Antibodies (basel) 5:19. https://doi.org/10.3390/antib5030019
Li YM, Tian ZW, Xu DH, Wang XY, Wang TY (2018) Construction strategies for developing expression vectors for recombinant monoclonal antibody production in CHO cells. Mol Biol Rep 45:2907–2912. https://doi.org/10.1007/s11033-018-4351-0
Li H, Er Saw P, Song E (2020) Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol Immunol 17:451–461. https://doi.org/10.1038/s41423-020-0417-8
Li YM, Wang M, Wang TY, Wei YG, Guo X, Mi CL, Zhao CP, Cao XX, Dou YY (2020) Effects of different 2A peptides on transgene expression mediated by tricistronic vectors in transfected CHO cells. Mol Biol Rep 47:469–475. https://doi.org/10.1007/s11033-019-05153-3
Li WF, Fan ZL, Lin Y, Wang TY (2021) Serum-free medium for recombinant protein expression in Chinese hamster ovary cells. Front Bioeng Biotechnol 9:646363. https://doi.org/10.3389/fbioe.2021.646363
Liu X, Chen Y, Zhao Y, Liu-Compton V, Chen W, Payne G, Lazar AC (2019) Identification and characterization of co-purifying CHO host cell proteins in monoclonal antibody purification process. J Pharm Biomed Anal 174:500–508. https://doi.org/10.1016/j.jpba.2019.06.021
Mamipour M, Yousefi M, Hasanzadeh M (2017) An overview on molecular chaperones enhancing solubility of expressed recombinant proteins with correct folding. Int J Biol Macromol 102:367–375. https://doi.org/10.1016/j.ijbiomac.2017.04.025
Martínez-Salas E (1999) Internal ribosome entry site biology and its use in expression vectors. Curr Opin Biotechnol 10:458–464. https://doi.org/10.1016/s0958-1669(99)00010-5
Mathias S, Wippermann A, Raab N, Zeh N, Handrick R, Gorr I, Schulz P, Fischer S, Gamer M, Otte K (2020) Unraveling what makes a monoclonal antibody difficult-to-express: from intracellular accumulation to incomplete folding and degradation via ERAD. Biotechnol Bioeng 117:5–16. https://doi.org/10.1002/bit.27196
Mohan C, Lee GM (2010) Effect of inducible co-overexpression of protein disulfide isomerase and endoplasmic reticulum oxidoreductase on the specific antibody productivity of recombinant Chinese hamster ovary cells. Biotechnol Bioeng 107:337–346. https://doi.org/10.1002/bit.22781
Mohan C, Park SH, Chung JY, Lee GM (2007) Effect of doxycycline-regulated protein disulfide isomerase expression on the specific productivity of recombinant CHO cells: thrombopoietin and antibody. Biotechnol Bioeng 98:611–615. https://doi.org/10.1002/bit.21453
Ng SK, Lin W, Sachdeva R, Wang DI, Yap MG (2010) Vector fragmentation: characterizing vector integrity in transfected clones by Southern blotting. Biotechnol Prog 26:11–20. https://doi.org/10.1002/btpr.281
Nishi H, Miyajima M, Nakagami H, Noda M, Uchiyama S, Fukui K (2010) Phase separation of an IgG1 antibody solution under a low ionic strength condition. Pharm Res 27:1348–1360. https://doi.org/10.1007/s11095-010-0125-7
Nishi H, Miyajima M, Wakiyama N, Kubota K, Hasegawa J, Uchiyama S, Fukui K (2011) Fc domain mediated self-association of an IgG1 monoclonal antibody under a low ionic strength condition. J Biosci Bioeng 112:326–332. https://doi.org/10.1016/j.jbiosc.2011.06.017
Ohtake S, Wang YJ (2011) Trehalose: current use and future applications. J Pharm Sci 100:2020–2053. https://doi.org/10.1002/jps.22458
Oliva A, Fariña JB, Llabrés M (2016) Pre-study and in-study validation of a size-exclusion chromatography method with different detection modes for the analysis of monoclonal antibody aggregates. J Chromatogr B Analyt Technol Biomed Life Sci 1022:206–212. https://doi.org/10.1016/j.jchromb.2016.04.022
Onitsuka M, Tatsuzawa M, Asano R, Kumagai I, Shirai A, Maseda H, Omasa T (2014) Trehalose suppresses antibody aggregation during the culture of Chinese hamster ovary cells. J Biosci Bioeng 117:632–638. https://doi.org/10.1016/j.jbiosc.2013.10.022
Paul AJ, Handrick R, Ebert S, Hesse F (2018) Identification of process conditions influencing protein aggregation in Chinese hamster ovary cell culture. Biotechnol Bioeng 115:1173–1185. https://doi.org/10.1002/bit.26534
Philo JS (2006) Is any measurement method optimal for all aggregate sizes and types? Aaps j 8:E564–E571. https://doi.org/10.1208/aapsj080365
Rader C (2020) Bispecific antibodies in cancer immunotherapy. Curr Opin Biotechnol 65:9–16. https://doi.org/10.1016/j.copbio.2019.11.020
Reichert D, Gröger S, Hackel C (2017) New insights into the interaction of proteins and disaccharides-the effect of pH and concentration. Biopolymers 107:39–45. https://doi.org/10.1002/bip.22990
Reinhart D, Damjanovic L, Kaisermayer C, Sommeregger W, Gili A, Gasselhuber B, Castan A, Mayrhofer P, Grünwald-Gruber C, Kunert R (2019) Bioprocessing of recombinant CHO-K1, CHO-DG44, and CHO-S: CHO expression hosts favor either mAb production or biomass synthesis. Biotechnol J 14:e1700686. https://doi.org/10.1002/biot.201700686
Rita Costa A, Elisa Rodrigues M, Henriques M, Azeredo J, Oliveira R (2010) Guidelines to cell engineering for monoclonal antibody production. Eur J Pharm Biopharm 74:127–138. https://doi.org/10.1016/j.ejpb.2009.10.002
Roberts CJ (2014) Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol 32:372–380. https://doi.org/10.1016/j.tibtech.2014.05.005
Rosenberg AS (2006) Effects of protein aggregates: an immunologic perspective. Aaps j 8:E501-507. https://doi.org/10.1208/aapsj080359
Schanzer JM, Wartha K, Croasdale R, Moser S, Künkele KP, Ries C, Scheuer W, Duerr H, Pompiati S, Pollman J, Stracke J, Lau W, Ries S, Brinkmann U, Klein C, Umana P (2014) A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties. J Biol Chem 289:18693–18706. https://doi.org/10.1074/jbc.M113.528109
Schatz SM, Kerschbaumer RJ, Gerstenbauer G, Kral M, Dorner F, Scheiflinger F (2003) Higher expression of Fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng 84:433–438. https://doi.org/10.1002/bit.10793
Schlatter S, Stansfield SH, Dinnis DM, Racher AJ, Birch JR, James DC (2005) On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells. Biotechnol Prog 21:122–133. https://doi.org/10.1021/bp049780w
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360. https://doi.org/10.1038/nrm.2017.20
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA (2018) Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther 12:195–208. https://doi.org/10.2147/dddt.s151282
Senga Y, Doi M, Onitsuka M, Honda S (2021) Live-cell imaging to analyze intracellular aggregation of recombinant IgG in CHO cells. Cell Chem Biol. https://doi.org/10.1016/j.chembiol.2021.08.010
Sinharoy P, Aziz AH, Majewska NI, Ahuja S, Handlogten MW (2020) Perfusion reduces bispecific antibody aggregation via mitigating mitochondrial dysfunction-induced glutathione oxidation and ER stress in CHO cells. Sci Rep 10:16620. https://doi.org/10.1038/s41598-020-73573-4
Stolfa G, Smonskey MT, Boniface R, Hachmann AB, Gulde P, Joshi AD, Pierce AP, Jacobia SJ, Campbell A (2018) CHO-Omics review: the impact of current and emerging technologies on Chinese hamster ovary based bioproduction. Biotechnol J 13:e1700227. https://doi.org/10.1002/biot.201700227
Swope N, Chung WK, Cao M, Motabar D, Liu D, Ahuja S, Handlogten M (2020) Impact of enzymatic reduction on bivalent bispecific antibody fragmentation and loss of product purity upon reoxidation. Biotechnol Bioeng 117:1063–1071. https://doi.org/10.1002/bit.27264
Tan JG, Lee YY, Wang T, Yap MG, Tan TW, Ng SK (2015) Heat shock protein 27 overexpression in CHO cells modulates apoptosis pathways and delays activation of caspases to improve recombinant monoclonal antibody titre in fed-batch bioreactors. Biotechnol J 10:790–800. https://doi.org/10.1002/biot.201400764
Telikepalli SN, Kumru OS, Kalonia C, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB (2014) Structural characterization of IgG1 mAb aggregates and particles generated under various stress conditions. J Pharm Sci 103:796–809. https://doi.org/10.1002/jps.23839
Vázquez-Rey M, Lang DA (2011) Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng 108:1494–1508. https://doi.org/10.1002/bit.23155
Wälchli R, Ressurreição M, Vogg S, Feidl F, Angelo J, Xu X, Ghose S, Jian Li Z, Le Saoût X, Souquet J, Broly H, Morbidelli M (2020) Understanding mAb aggregation during low pH viral inactivation and subsequent neutralization. Biotechnol Bioeng 117:687–700. https://doi.org/10.1002/bit.27237
Wälchli R, Vermeire PJ, Massant J, Arosio P (2020) Accelerated aggregation studies of monoclonal antibodies: considerations for storage stability. J Pharm Sci 109:595–602. https://doi.org/10.1016/j.xphs.2019.10.048
Wang TY, Guo X (2020) Expression vector cassette engineering for recombinant therapeutic production in mammalian cell systems. Appl Microbiol Biotechnol 104:5673–5688. https://doi.org/10.1007/s00253-020-10640-w
Wang W, Roberts CJ (2013) Non-Arrhenius Protein Aggregation. Aaps j 15:840–851. https://doi.org/10.1208/s12248-013-9485-3
Wang W, Roberts CJ (2018) Protein aggregation - mechanisms, detection, and control. Int J Pharm 550:251–268. https://doi.org/10.1016/j.ijpharm.2018.08.043
Wang Q, Chung CY, Chough S, Betenbaugh MJ (2018) Antibody glycoengineering strategies in mammalian cells. Biotechnol Bioeng 115:1378–1393. https://doi.org/10.1002/bit.26567
Wang Q, Chen Y, Park J, Liu X, Hu Y, Wang T, McFarland K, Betenbaugh MJ (2019) Design and production of bispecific antibodies. Antibodies (basel) 8:43. https://doi.org/10.3390/antib8030043
Wang Z, Zhu J, Lu H (2020) Antibody glycosylation: impact on antibody drug characteristics and quality control. Appl Microbiol Biotechnol 104:1905–1914. https://doi.org/10.1007/s00253-020-10368-7
Wranik BJ, Christensen EL, Schaefer G, Jackman JK, Vendel AC, Eaton D (2012) LUZ-Y, a novel platform for the mammalian cell production of full-length IgG-bispecific antibodies. J Biol Chem 287:43331–43339. https://doi.org/10.1074/jbc.M112.397869
Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, Jacobs SA, Teplyakov A, Gilliland GL, Feng Y (2010) Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel 23:643–651. https://doi.org/10.1093/protein/gzq037
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. https://doi.org/10.1172/jci26373
Yageta S, Lauer TM, Trout BL, Honda S (2015) Conformational and colloidal stabilities of isolated constant domains of human immunoglobulin G and their impact on antibody aggregation under acidic conditions. Mol Pharm 12:1443–1455. https://doi.org/10.1021/mp500759p
Yeo JHM, Mariati YY (2018) An IRES-mediated tricistronic vector for efficient generation of stable, high-level monoclonal antibody producing CHO DG44 cell lines. Methods Mol Biol 1827:335–349. https://doi.org/10.1007/978-1-4939-8648-4_17
Yue YL, Yin J, Gao XD, Yao WB (2019) Research advances of bispecific antibody drugs in tumor therapy. J China Pharm Univ 50:289–298. https://doi.org/10.11665/j.issn.1000-5048.20190304
Zhang W, Liu X, Tang H, Zhang X, Zhou Y, Fan L, Wang H, Tan WS, Zhao L (2020) Investigation into the impact of tyrosine on the product formation and quality attributes of mAbs in rCHO cell cultures. Appl Microbiol Biotechnol 104:6953–6966. https://doi.org/10.1007/s00253-020-10744-3
Zheng K, Ren D, Wang YJ, Lilyestrom W, Scherer T, Hong JKY, Ji JA (2021) Monoclonal antibody aggregation associated with free radical induced oxidation. Int J Mol Sci 22:https://doi.org/10.3390/ijms22083952
Acknowledgements
The authors thank Zhao-Hui Zhang and Editage for the English language editing of the document.
Funding
This work was funded by the Basic Research Foundation of Key Scientific Research of Universities in Henan Province (No.20zx013), the Key Scientific Research Project of Higher Education of Henan Province, China (No.22A310009), and the Natural Science Foundation of Henan province (No. 212300410384).
Author information
Authors and Affiliations
Contributions
Ting-Ting Xu and Tian-Yun Wang wrote and designed the manuscript; Ji-Hong Zhang and Xiao-Yin Wang edited the manuscript; and Tian-Yun Wang designed the content of the review and funded this manuscript.
Corresponding authors
Ethics declarations
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, T., Zhang, J., Wang, T. et al. Recombinant antibodies aggregation and overcoming strategies in CHO cells. Appl Microbiol Biotechnol 106, 3913–3922 (2022). https://doi.org/10.1007/s00253-022-11977-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11977-0