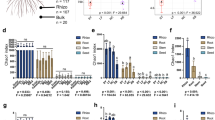
Abstract
The plant’s endophytic fungi play an important role in promoting host development and metabolism. Studies have shown that the factors affecting the assembly of the endophyte community mainly include host genotype, vertical transmission, and soil origin. However, we do not know the role of vertically transmitted endohytic fungi influences on the host-plant’s endophytic community assembly. Salvia miltiorrhiza from three production areas were used as research objects; we constructed three production area genotypes of S. miltiorrhiza regenerated seedlings simultaneously. Based on high-throughput sequencing, we analyzed the effects of genotype, soil origin, and vertical transmission on endophytic fungal communities. The results show that the community of soil origins significantly affected the endophytic fungal community in the regenerated seedlings of S. miltiorrhiza. The influence of genotype on community composition occurs through a specific mechanism. Genotype may selectively screen certain communities into the seed, thereby exerting selection pressure on the community composition process of offspring. As the number of offspring increases gradually, the microbiota, controlled by genotype and transmitted vertically, stabilizes, ultimately resulting in a significant effect of genotype on community composition.
Furthermore, we observed that the taxa influencing the active ingredients are also selected as the vertically transmitted community. Moreover, the absence of an initial vertically transmitted community in S. miltiorrhiza makes it more vulnerable to infection by pathogenic fungi. Therefore, it is crucial to investigate and comprehend the selection model of the vertically transmitted community under varying genotypes and soil conditions. This research holds significant implications for enhancing the quality and yield of medicinal plants and economic crops.



Similar content being viewed by others
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI (accession: PRJNA756137 & PRJNA761573).
References
Deng Z, Cao L (2017) Fungal endophytes and their interactions with plants in phytoremediation: a review. Chemosphere 168:1100–1106
Mejía Luis C, Herre EA, Sparks JP, Winter K, García Miltion N, Bael Sunshine A, Van SJ, Shi Z, Zhang Y, Guiltinan Mark J, Maximova Siela N (2014) Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol 5:479
Yan L, Zhu J, Zhao X, Shi J, Jiang C, Shao D (2019) Beneficial effects of endophytic fungi colonization on plants. Appl Microbiol Biotechnol 103(8):3327–3340
del Pilar Martínez-Diz M, Andrés-Sodupe M, Bujanda R, Díaz-Losada E, Eichmeier A, Gramaje D (2019) Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol 41:234–244
Latz MAC, Kerrn MH, Sørensen H, Collinge DB, Jensen B, Brown JKM, Madsen AMM, Jørgensen HJL (2021) Succession of the fungal endophytic microbiome of wheat is dependent on tissue-specific interactions between host genotype and environment. Sci Total Environ 759:143804
Chen H, Wu H, Yan B, Zhao H, Liu F, Zhang H, Sheng Q, Miao F, Liang Z (2018) Core microbiome of medicinal plant Salvia miltiorrhiza seed: a rich reservoir of beneficial microbes for secondary metabolism? Int J Mol Sci 19(3):672
Leopold DR, Busby PE (2020) Host genotype and colonist arrival order jointly govern plant microbiome composition and function. Curr Biol 30(16):3260–3266.e5
Cooper M, van Eeuwijk FA, Hammer GL, Podlich DW, Messina C (2009) Modeling QTL for complex traits: detection and context for plant breeding. Curr Opin Plant Biol 12(2):231–240
Yan J, Zhu J, He C, Benmoussa M, Ping W (1999) Molecular marker-assisted dissection of genotype × environment interaction for plant type traits in rice (Oryza sativa L.). Crop Sci 39(2):538–544
Huang W, Long C, Lam E (2018) Roles of plant-associated microbiota in traditional herbal medicine. Trends Plant Sci 23(7):559–562
Shao F, Lu S (2013) Genome-wide identification, molecular cloning, expression profiling and posttranscriptional regulation analysis of the Argonaute gene family in Salvia miltiorrhiza, an emerging model medicinal plant. BMC Genomics 14:1–12
Fu J, Huang H, Liu J, Pi R, Chen J, Liu P (2007) Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 568(1-3):213–221
Zhang J, Jin Q, Deng Y, Hou J, Wu W, Guo D (2017) New depsides from the roots of Salvia miltiorrhiza and their radical-scavenging capacity and protective effects against H2O2-induced H9c2 cells. Fitoterapia 121:46–52
Pan Y, Fu H, Kong Q, Xiao Y, Shou Q, Chen H, Ke Y, Chen M (2014) Prevention of pulmonary fibrosis with salvianolic acid a by inducing fibroblast cell cycle arrest and promoting apoptosis. J Ethnopharmacol 155(3):1589–1596
Tang H, He H, Ji H, Gao L, Mao J, Liu J, Lin H, Wu T (2015) Tanshinone IIA ameliorates bleomycin-induced pulmonary fibrosis and inhibits transforming growth factor-beta-β–dependent epithelial to mesenchymal transition. J Surg Res 197(1):167–175
Liu H, Ma S, Xia H, Lou H, Zhu F, Sun L (2018) Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J Ethnopharmacol 222:201–207
Xu X, Lv H, Li X, Su H, Zhang X, Yang J (2017) Danshen attenuates osteoarthritis-related cartilage degeneration through inhibition of NF-κB signaling pathway in vivo and in vitro. Biochem Cell Biol 95(6):644–651
Zhang D-W, Liu X, Xie D, Chen R, Tao X-Y, Zou J-H, Dai J (2013) Two new diterpenoids from cell cultures of Salvia miltiorrhiza. Chem Pharm Bull 61(5):576–580
Jiang G, Liu J, Ren B, Zhang L, Owusu L, Liu L, Zhang J, Tang Y, Li W (2017) Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J Ethnopharmacol 205:33–40
Wang X, Gao A, Jiao Y, Zhao Y, Yang X (2018) Antitumor effect and molecular mechanism of antioxidant polysaccharides from Salvia miltiorrhiza Bunge in human colorectal carcinoma LoVo cells. Int J Biol Macromol 108:625–634
Wang Y, Cao J (2016) Advances in the chemical and pharmacological studies of phenolic acids in Salvia miltiorrhiza. World Chin Med 11(6):1126–1130
Xing B, Liang L, Liu L, Hou Z, Yang D, Yan K, Zhang X, Liang Z (2018) Overexpression of SmbHLH148 induced biosynthesis of tanshinones as well as phenolic acids in Salvia miltiorrhiza hairy roots. Plant Cell Rep 37(12):1681–1692
Dong Y, Morris-Natschke SL, Lee K-H (2011) Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat Prod Rep 28(3):529–542
Zhou L, Zuo Z, Chow MSS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45(12):1345–1359
Sun J, Xia F, Cui L, Liang J, Wang Z, Wei Y (2014) Characteristics of foliar fungal endophyte assemblages and host effective components in Salvia miltiorrhiza Bunge. Appl Microbiol Biotechnol 98(7):3143–3155
Teimoori-Boghsani Y, Ganjeali A, Cernava T, Müller H, Asili J, Berg G (2020) Endophytic fungi of native Salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front Microbiol 10:3013
Zhai X, Luo D, Li X, Han T, Jia M, Kong Z, Ji J, Rahman K, Qin L, Zheng C (2018) Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front Microbiol 8:2694
Ernst M, Mendgen KW, Wirsel SG (2003) Endophytic fungal mutualists: seed-borne Stagonospora spp. enhance reed biomass production in axenic microcosms. Mol Plant-Microbe Interact 16(7):580–587
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Xu G, Liu C, Huang L, Wang X, Zhang Y, Liu S, Liao C, Yuan Q, Zhou X (2013) Development of new EST-derived SSRs in Salvia miltiorrhiza (Labiatae) in China and preliminary analysis of genetic diversity and population structure. Biochem Syst Ecol 51:308–313
Lan Y, Liu M, Yan Z, Xie H, Sheng X, Zhang L, Lin C (2016a) Comparison of the characteristics of plant cloning and regeneration seedlings in different geographical sources. Jiangsu Agri Sci 44(10):103–103
Lan Y, Sheng X, Yan Z, Wang H, He D, Wang S, Red B, Chen X (2016b) Comparative analysis of GC-MS of root exudates in salvia miltiorriza from different geographic sources. Jiangsu Agri Sci 44(1):301–305
Wang M, Dai G, Ma Y, Zhang Q, Chen X, Wan DG, Yan Z (2013) Study on correlation between active components and endophytic fungi in salvia miltiorriza. Chin J Exp Tradit Med Formulae 19(23):66–73
Borneman J, Hartin RJ (2000) PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66(10):4356–4360
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47(D1):D259–D264
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Team, R. C. (2013). “R: A language and environment for statistical computing.”
Stopnisek N, Shade A (2021) Persistent microbiome members in the common bean rhizosphere: an integrated analysis of space, time, and plant genotype. ISME J 15(9):2708–2722
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan’. Comm Ecol Package 2(9):1–295
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Kolde R, Kolde MR (2015) Package ‘pheatmap’. R package 1(7):790
Wickham H (2011) ggplot2. Wiley Interdiscipl Rev : comput Stat 3(2):180–185
Putten P (1979) Mandelic acid and urinary tract infections. Antonie Van Leeuwenhoek 45(4):622–623
Li X, He C, He X, Su F, Hou L, Ren Y, Hou Y (2019) Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 439(1):259–272
Al-Hosni K, Shahzad R, Latif Khan A, Muhammad Imran Q, Al Harrasi A, Al Rawahi A, Asaf S, Kang S-M, Yun B-W, Lee I-J (2018) Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J Plant Interact 13(1):112–118
Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A, Waqas M, Asaf S, Elyassi A, Mabood F, Shin J-H (2016) Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS One 11(6):e0158207
Wang M, Xue J, Ma J, Feng X, Ying H, Xu H (2020) Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front Microbiol 11:942
Mapperson RR, Kotiw M, Davis RA, Dearnaley JD (2014) The diversity and antimicrobial activity of Preussia sp. endophytes isolated from Australian dry rainforests. Curr Microbiol 68(1):30–37
Farh ME-A, Kim Y-J, Kim Y-J, Yang D-C (2018) Cylindrocarpon destructans/Ilyonectria radicicola-species complex: causative agent of ginseng root-rot disease and rusty symptoms. J Ginseng Res 42(1):9–15
Fassatiová O (1983) Grzyby mikroskopowe w mikrobiologii technicznej. Wydawnictwa Naukowo-Techniczne, Poland
Gilbert J, Tekauz A, Woods S (1995) Effect of Phaeosphaeria nodorum-induced seed shrivelling on subsequent wheat emergence and plant growth. Euphytica 82(1):9–16
Li C, Wu Y, Li L, Zhao C, Li B, Wu Y, Wang H, Yan Z (2023) Different techniques reveal the difference of community structure and function of fungi from root and rhizosphere of Salvia miltiorrhiza Bunge. Plant Biol. https://doi.org/10.1111/plb.13556
Challacombe JF, Hesse CN, Bramer LM, McCue LA, Lipton M, Purvine M, Nicora C, Gallegos-Graves LV, Porras-Alfaro A, Kuske CR (2019) Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genomics 20(1):1–27
Eo JK, Eom AH (2022) Community of endophytic fungi from alpine conifers on Mt. Seorak. Mycobiology 50(5):317–325
Giampetruzzi A, Baptista P, Morelli M, Cameirão C, Lino Neto T, Costa D, D’Attoma G, Abou Kubaa R, Altamura G, Saponari M, Pereira JA, Saldarelli P (2020) Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across seasons. Pathogens 9(9):723
Lê VA, Quaiser A, Duhamel M, Michon-Coudouel S, Dufresne A, Vandenkoornhuyse P (2017) Ecophylogeny of the endospheric root fungal microbiome of co-occurring Agrostis stolonifera. PeerJ 5:e3454
Bulgarelli D, Rott M, Schlaeppi K, Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488(7409):91–95
Dombrowski N, Schlaeppi K, Agler MT, Hacquard S, Kemen E, Garrido-Oter R, Wunder J, Coupland G, Schulze-Lefer P (2017) Root microbiota dynamics of perennial Arabis alpina are dependent on soil residence time but independent of flowering time. ISME J 11(1):43–55
Schlaeppi K, Dombrowski N, Oter RG, Themaat EVL, Schulze-Lefert P (2014) Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc Natl Acad Sci 111(2):585–592
Chen D, Jia L, Hou Q, Zhao X, Sun K (2021) Analysis of endophyte diversity of Rheum palmatum from different production areas in Gansu province of China and the association with secondary metabolite. Microorganisms 9(5):978
Wang R, Zhang Q, Ju M, Yan S, Zhang Q, Gu P (2011) The endophytic fungi diversity, community structure, and ecological function prediction of Sophora alopecuroides in Ningxia, China. Microorganisms 10(11):2099
Yan K, Pei Z, Meng L, Zheng Y, Wang L, Feng R, Li Q, Liu Y, Zhao X, Wei Q, El-Sappah AH, Abbas M (2022) Determination of community structure and diversity of seed-vectored endophytic fungi in Alpinia zerumbet. Front Microbiol 13:814864
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J, Medema MH, Maltz MR, Mundra S, Olsson PA, Pent M, Põlme S, Sunagawa S, Ryberg M et al (2018) Structure and function of the global topsoil microbiome. Nature 560(7717):233–237
Egidi E, Delgado-Baquerizo M, Plett JM, Wang J, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK (2019) A few Ascomycota taxa dominate soil fungal communities worldwide. Nat Commun 10(1):2369
Răut I, Călin M, Capră L, Gurban A, Doni M, Radu N, Jecu L (2021) Cladosporium sp. isolate as fungal plant growth promoting agent. Agronomy 11(2):392
Lou J, Fu L, Peng Y, Zhou L (2013) Metabolites from Alternaria fungi and their bioactivities. Molecules 18(5):5891–5935
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8(9):e71805
Martinez-Klimova E, Rodríguez-Peña K, Sánchez S (2017) Endophytes as sources of antibiotics. Biochem Pharmacol 134:1–17
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M (2005) An endophytic fungus from Nothapodytes f oetida that produces Camptothecin. J Nat Prod 68(12):1717–1719
East R (2013) Microbiome: Soil science comes to life. Nature 501(7468):S18–S19
Zarraonaindia I, Owens SM, Weisenhorn P, West K, Hampton-Marcell J, Lax S, Bokulich NA, Mills DA, Martin G, Taghavi S, Lelie DVD, Gilbert JA, Jansson JK (2015) The soil microbiome influences grapevine-associated microbiota. mBio 6(2):e02527–e02514
He C, Cui J, Chen X, Wang W, Hou J (2020) Effects of enhancement of liquorice plants with dark septate endophytes on the root growth, glycyrrhizic acid and glycyrrhizin accumulation amended with organic residues. Curr Plant Biol 23:100154
He C, Wang W, Hou J (2019) Characterization of dark septate endophytic fungi and improve the performance of liquorice under organic residue treatment. Front Microbiol 10:1364
Kumar R, Kundu A, Dutta A, Saha S, Das A, Bhowmik A (2021) Chemo-profiling of bioactive metabolites from Chaetomium globosum for biocontrol of Sclerotinia rot and plant growth promotion. Fungal Biol 125(3):167–176
Xu XD, Liang W-X, Yao L, Paek K-Y, Wang J, Gao W-Y (2021) Production of ginsenoside by Chaetomium sp. and its effect on enhancing the contents of ginsenosides in Panax ginseng adventitious roots. Biochem Eng J 174:108100
Kuchkarova N, Toshmatov Z, Zhou S, Han C, Shao H (2020) Secondary metabolites with plant growth regulator activity produced by an endophytic fungus Purpureocillium sp. from Solanum rostratum. Chem Nat Compd 56(4):775–776
Schulz B, Boyle C, Draeger S, Römmert A-K, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites* *Paper presented at the British Mycological Society symposium on Fungal Bioactive Compounds, held at the University of Wales Swansea on 22–27 April 2001. Mycol Res 106(9):996–1004
Zhou LS, Tang K, Guo SX (2018) The plant growth-promoting fungus (PGPF) Alternaria sp. A13 markedly enhances Salvia miltiorrhiza root growth and active ingredient accumulation under greenhouse and field conditions. Int J Mol Sci 19(1):270
Khushdil F, Jan FG, Jan G, Hamayun M, Iqbal A, Hussain A, Bibi N (2019) Salt stress alleviation in Pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus. Plant Physiol Biochem 144:127–134
Lubna S, Asaf M, Hamayun H, Gul I-JL, Hussain A (2018) Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J Plant Interact 13(1):100–111
Amprayn KO, Rose MT, Pereg K, Nguyen HT, Kennedy and IR (2012) Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl Soil Ecol 61:295–299
Silambarasan S, Vangnai AS (2017) Plant-growth promoting Candida sp. AVGB4 with capability of 4-nitroaniline biodegradation under drought stress. Ecotoxicol Environ Saf 139:472–480
Stosiek N, Terebieniec A, Ząbek A, Młynarz P, Cieśliński H, Klimek-Ochab M (2019) N-phosphonomethylglycine utilization by the psychrotolerant yeast Solicoccozyma terricola M 3.1. 4. Bioorg Chem 93:102866
He J-W, Chen G-D, Gao H, Yang F, Li X-X, Peng T, Guo L-D, Yao X-S (2012) Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 83(6):1087–1091
Kellogg JJ, Raja HA (2017) Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon. Phytochem Rev 16(2):271–293
Yeganeh T-B, Ganjeali A, Cernava T, Müller H, Javad, Asili (2019) Endophytic fungi of native salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front Microbiol 10:3013–3013
Chang HS, Cheng MJ, Wu M, Chan HY, Hsieh SY, Lin CH, Yech YJ, Chen IS (2017) Secondary metabolites produced by an endophytic fungus Cordyceps ninchukispora from the seeds of Beilschmiedia erythrophloia Hayata. Phytochem Lett 22:179–184
Zhou X, Gong Z, Su Y, Lin J, Tang K (2009) Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol 61(3):279–291
Shrestha B, Zhang W, Zhang Y, Liu X (2010) What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? Mycology 1(4):228–236
Zjalic S, Reverberi M, Ricelli A, Granito VM, Fanelli C, Fabbri AA (2006) Trametes versicolor: a possible tool for aflatoxin control. Int J Food Microbiol 107(3):243–249
Vujanovic V (2021) Tremellomycetes yeasts in kernel ecological niche: early indicators of enhanced competitiveness of endophytic and mycoparasitic symbionts against wheat pathobiota. Plants 10(5):905
Meteyer CU, Dutheil JY, Keel MK, Boyles JG, Stukenbrock EH (2022) Plant pathogens provide clues to the potential origin of bat white-nose syndrome Pseudogymnoascus destructans. Virulence 13(1):1020–1031
Saunte DML, Gaitanis G, Hay RJ (2020) Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol 10:112
Bonito G, Reynolds H, Robeson MS, Nelson J, Hodkinson BP, Tuskan G, Schadt CW, Vilgalys R (2014) Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol Ecol 23(13):3356–3370
Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, North G, Visel A, Partida-Martinez L, Tringe SG (2016) Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol 209(2):798–811
Cregger MA, Veach AM, Yang ZK, Crouch MJ, Vilgalys R, Tuskan GA, Schadt CW (2018) The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome. Microbiome 6(1):1–14
Wagner MR, Lundberg DS, del Rio TG, Tringe SG, Dangl JL, Mitchell-Olds T (2016) Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat Commun 7(1):12151
Glynou K, Ali T, Buch AK, Kia SH, Ploch S, Xia X, Elik A, Thines M, Maciá-Vicente J (2016) The local environment determines the assembly of root endophytic fungi at a continental scale. Environ Microbiol 18(8):2418–2434
Faddetta T, Abbate L, Alibrandi P, Arancio W, Siino D, Strati F, De Filippo C, Del Bosco SF, Carimi F, Puglia AM (2021) The endophytic microbiota of Citrus limon is transmitted from seed to shoot highlighting differences of bacterial and fungal community structures. Sci Rep 11(1):1–12
Vannier N, Mony C, Bittebiere A, Michon-Coudouel S, Biget M, Vandenkoornhuyse P (2018) A microorganisms’ journey between plant generations. Microbiome 6(1):1–11
Zhang X, Ma Y, Wang X, Liao K, He S, Zhao X, Guo H, Zhao DWH (2022) Dynamics of rice microbiomes reveal core vertically transmitted seed endophytes. Microbiome 10(1):1–19
Nelson EB (2018) The seed microbiome: origins, interactions, and impacts. Plant Soil 422(1):7–34
Shade A, Jacques M-A, Barret M (2017) Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr Opin Microbiol 37:15–22
Griffiths SM, Antwis RE, Lenzi L, Lucaci A, Behringer DC, Butler MJ, Preziosi RF (2019) Host genetics and geography influence microbiome composition in the sponge Ircinia campana. J Anim Ecol 88(11):1684–1695
Busby PE, Newcombe G, Neat AS, Averill C (2022) Facilitating reforestation through the plant microbiome: perspectives from the phyllosphere. Annu Rev Phytopathol 60:337–356
Funding
This study was supported by grants from the National Natural Science Foundation of China(81173493).
Author information
Authors and Affiliations
Contributions
Hongyang Lv and Zhuyun Yan contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jin Zhao, Hai Wang, Dongmei He, Xin Chen, Yin Lan and Min Liu. The first draft of the manuscript was written by Hongyang Lv and Xiaoyu Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, H., Li, X., He, D. et al. Genotype-Controlled Vertical Transmission Exerts Selective Pressure on Community Assembly of Salvia miltiorrhiza. Microb Ecol 86, 2934–2948 (2023). https://doi.org/10.1007/s00248-023-02295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02295-7