Abstract
Purpose
Pemigatinib (INCB054828), a potent and selective oral fibroblast growth factor receptor 1–3 inhibitor, is a Biopharmaceutical Classification System class II compound with good permeability and pH-dependent solubility that is predominantly metabolized by cytochrome P450 (CYP) 3A. Two drug-drug interaction studies, one with acid-reducing agents, esomeprazole (proton pump inhibitor [PPI]) and ranitidine (histamine-2 [H2] antagonist), and the other with potent CYP3A-modulating agents, itraconazole (CYP3A inhibitor) and rifampin (CYP3A inducer), were performed.
Methods
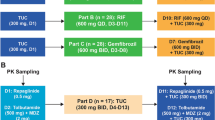
Both were open-label, fixed-sequence studies conducted in up to 36 healthy participants each, enrolled into two cohorts (n = 18 each). Pemigatinib plasma concentration was measured, and pharmacokinetic parameters were derived by non-compartmental analysis.
Results
There was an 88% and 17% increase in pemigatinib area under the plasma drug concentration–time curve (AUC) and maximum plasma drug concentration (Cmax), respectively, with itraconazole, and an 85% and 62% decrease in pemigatinib AUC and Cmax with rifampin coadministration. There was a 35% and 8% decrease in pemigatinib AUC and Cmax, respectively, with esomeprazole, and a 2% decrease in Cmax and 3% increase in AUC with ranitidine coadministration. In both studies, all adverse events reported were grade ≤ 2.
Conclusion
Coadministration with itraconazole or rifampin resulted in a clinically significant change in pemigatinib exposure. Therefore, coadministration of strong CYP3A inducers with pemigatinib should be avoided, and the dose of pemigatinib should be reduced if coadministration with strong CYP3A inhibitors cannot be avoided. The effect of PPIs/H2 antagonists on pemigatinib exposure was modest, and pemigatinib can be administered without regard to coadministration of PPIs/H2 antagonists.




Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author (email: tji@incyte.com) on reasonable request.
Change history
26 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00228-021-03198-7
References
Liu PCC, Koblish H, Wu L, Bowman K, Diamond S, DiMatteo D, Zhang Y, Hansbury M, Rupar M, Wen X, Collier P, Feldman P, Klabe R, Burke KA, Soloviev M, Gardiner C, He X, Volgina A, Covington M, Ruggeri B, Wynn R, Burn TC, Scherle P, Yeleswaram S, Yao W, Huber R, Hollis G (2020) INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS One 15(4):e0231877. https://doi.org/10.1371/journal.pone.0231877
Knights V, Cook SJ (2010) De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther 125(1):105–117. https://doi.org/10.1016/j.pharmthera.2009.10.001
Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10(2):116–129. https://doi.org/10.1038/nrc2780
PEMAZYRE™ (2021) (pemigatinib) tablets [prescription information]. Wilmington, DE: Incyte Corporation. https://www.pemazyre.com/pdf/prescribing-information.pdf. Accessed February 15, 2021
European Medicines Agency (2021) Pemazyre (pemigatinib). https://www.ema.europa.eu/en/medicines/human/EPAR/pemazyre. Accessed July 12, 2021
Incyte (2021) Incyte announces approval of Pemazyre® (pemigatinib) in Japan for the treatment of patients with unresectable biliary tract cancer (BTC) with a fibroblast growth factor receptor 2 (FGFR2) fusion gene, worsening after cancer chemotherapy [press release]. Wilmington, DE: Incyte Corporation. https://investor.incyte.com/press-releases/press-releases/2021/Incyte-Announces-Approval-of-Pemazyre-pemigatinib-in-Japan-for-the-Treatment-of-Patients-with-Unresectable-Biliary-Tract-Cancer-BTC-with-a-Fibroblast-Growth-Factor-Receptor-2-FGFR2-Fusion-Gene-Worsening-After-Cancer-Chemotherapy/default.aspx. Accessed July 13, 2021
Hoy SM (2020) Pemigatinib: First Approval. Drugs 80(9):923–929. https://doi.org/10.1007/s40265-020-01330-y
Merz V, Zecchetto C, Melisi D (2021) Pemigatinib, a potent inhibitor of FGFRs for the treatment of cholangiocarcinoma. Future Oncol 17(4):389–402. https://doi.org/10.2217/fon-2020-0726C
Rizzo A, Ricci AD, Brandi G (2021) Pemigatinib: hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun 27:100337. https://doi.org/10.1016/j.ctarc.2021.100337
Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, Melisi D, Macarulla T, Bridgewater J, Wasan H, Borad MJ, Abou-Alfa GK, Jiang P, Lihou CF, Zhen H, Asatiani E, Féliz L, Vogel A (2020) FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol 16(30):2385–2399. https://doi.org/10.2217/fon-2020-0429
Ji T, Lihou C, Asatiani E, Féliz L, Overholt H, Landman R, Chen X, Yeleswaram S (2019) Abstract C071: pharmacokinetics and pharmacodynamics of pemigatinib, a potent and selective inhibitor of FGFR 1, 2, and 3, in patients with advanced malignancies. Molecular Cancer Therapeutics 18(12 Supplement):C071–C071. https://doi.org/10.1158/1535-7163.Targ-19-c071
US Food and Drug Administration (2017) Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical. Accessed February 15, 2021
Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK (1999) The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 104(2):147–153. https://doi.org/10.1172/JCI6663
Ji T, Zhang Y, Overholt H, Chen X, Yeleswaram S (2020) Evaluation of CYP3A4-mediated and transporter-mediated drug-drug interaction potential for pemigatinib using physiologically-based pharmacokinetic modeling. Clin Pharmacol Ther 107: Abstract PII-123. https://doi.org/10.1002/cpt.1732
Acknowledgements
The authors thank the participants, investigators, and site personnel who participated in these studies. Editorial assistance was provided by Envision Pharma Group, Inc. (Philadelphia, PA, USA).
Funding
The study was funded by Incyte Corporation, Wilmington, DE, USA.
Author information
Authors and Affiliations
Contributions
Tao Ji, Naresh Punwani, Xuejun Chen, and Swamy Yeleswaram were involved in the conception/design of the work. Kevin Rockich, Noam Epstein, Heather Overholt, and Phillip Wang acquired the data. Tao Ji analyzed the data. All the authors drafted the manuscript or revised it critically for important intellectual content, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Both studies were performed in accordance with the International Council for Harmonization guideline for Good Clinical Practice, including the Declaration of Helsinki and local ethical and legal requirements.
Consent to participate
All participants provided written informed consent before initiating treatment.
Conflict of interest
Tao Ji, Kevin Rockich, Heather Overholt, Phillip Wang, Xuejun Chen, Naresh Punwani, and Swamy Yeleswaram are employees/stockholders of Incyte Corporation. Noam Epstein is an employee/stockholder of GlaxoSmithKline.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ji, T., Rockich, K., Epstein, N. et al. Evaluation of drug-drug interactions of pemigatinib in healthy participants. Eur J Clin Pharmacol 77, 1887–1897 (2021). https://doi.org/10.1007/s00228-021-03184-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03184-z