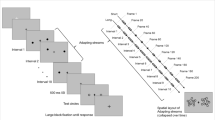
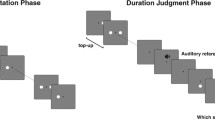
Abstract
The perceived duration of a target visual stimulus is shorter when a brief non-target visual stimulus precedes and trails the target than when it appears alone. This time compression requires spatiotemporal proximity of the target and non-target stimuli, which is one of the perceptual grouping rules. The present study examined whether and how another grouping rule, stimulus (dis)similarity, modulated this effect. In Experiment 1, time compression occurred only when the preceding and trailing stimuli (black–white checkerboard) were dissimilar from the target (unfilled round or triangle) with spatiotemporal proximity. In contrast, it was reduced when the preceding or trailing stimuli (filled rounds or triangles) were similar to the target. Experiment 2 revealed time compression with dissimilar stimuli, independent of the intensity or saliency of the target and non-target stimuli. Experiment 3 replicated the findings of Experiment 1 by manipulating the luminance similarity between target and non-target stimuli. Furthermore, time dilation occurred when the non-target stimuli were indistinguishable from the target stimuli. These results indicate that stimulus dissimilarity with spatiotemporal proximity induces time compression, whereas stimulus similarity with spatiotemporal proximity does not. These findings were discussed in relation to the neural readout model.






Similar content being viewed by others
Data availability
All datasets have been made publicly available at the OSF and can be accessed at [https://osf.io/hvmc6/]. The study design and analyses were not pre-registered.
References
Asaoka R (2020) Sandwiched visual stimuli are perceived as shorter than the stimulus alone. Acta Physiol (oxf) 203:102982
Asaoka R, Gyoba J (2016) Sounds modulate the perceived duration of visual stimuli via crossmodal integration. Multisens Res 29(4–5):319–335
Ben-Av MB, Sagi D (1995) Perceptual grouping by similarity and proximity: experimental results can be predicted by intensity autocorrelations. Vis Res 35(6):853–866
Born S, Krüger HM, Zimmermann E, Cavanagh P (2016) Compression of space for low visibility probes. Front Syst Neurosci 10:21
Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6(10):755–765
Buonomano DV, Merzenich MM (1995) Temporal information transformed into a spatial code by a neural network with realistic properties. Science 267(5200):1028–1030
Buonomano DV, Bramen J, Khodadadifar M (2009) Influence of the interstimulus interval on temporal processing and learning: testing the state-dependent network model. Philos Trans R Soc b: Biol Sci 364(1525):1865–1873
Burr D, Tozzi A, Morrone MC (2007) Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nat Neurosci 10(4):423–425
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale, pp 20–26
Derichs C, Zimmermann E (2016) Temporal binding of interval markers. Sci Rep 6(1):38806
Droit-Volet S, Wearden J (2002) Speeding up an internal clock in children? Effects of visual flicker on subjective duration. Q J Exp Psychol 55(3):193–211
Eagleman DM, Pariyadath V (2009) Is subjective duration a signature of coding efficiency. Philos Trans R Soc b: Biol Sci 364:1841–1851
Enns JT, Di Lollo V (2000) What’s new in visual masking? Trends Cogn Sci 4(9):345–352
Ernst B, Reichard SM, Riepl RF, Steinhauser R, Zimmermann SF, Steinhauser M (2017) The P3 and the subjective experience of time. Neuropsychologia 103:12–19
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Grondin S (2010) Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Atten Percept Psychophys 72(3):561–582
Han S, Humphreys GW (1999) Interactions between perceptual organization based on Gestalt laws and those based on hierarchical processing. Percept Psychophys 61(7):1287–1298
Han S, Song Y, Ding Y, Yund EW, Woods DL (2001) Neural substrates for visual perceptual grouping in humans. Psychophysiology 38(6):926–935
Han S, Jiang Y, Mao L, Humphreys GW, Gu H (2005) Attentional modulation of perceptual grouping in human visual cortex: Functional MRI studies. Hum Brain Mapp 25(4):424–432
Hayashi MJ, Ivry RB (2020) Duration selectivity in right parietal cortex reflects the subjective experience of time. J Neurosci 40(40):7749–7758
Jacobson JZ, Rhinelander G (1978) Geometric and semantic similarity in visual masking. J Exp Psychol Hum Percept Perform 4(2):224–231
Johnston A, Arnold DH, Nishida S (2006) Spatially localized distortions of event time. Curr Biol 16(5):472–479
Karmarkar UR, Buonomano DV (2007) Timing in the absence of clocks: encoding time in neural network states. Neuron 53(3):427–438
Lamme VA, Zipser K, Spekreijse H (2002) Masking interrupts figure-ground signals in V1. J Cogn Neurosci 14(7):1044–1053
Li B, Chen Y, Xiao L, Liu P, Huang X (2017) Duration adaptation modulates EEG correlates of subsequent temporal encoding. Neuroimage 147:143–151
Macknik SL, Livingstone MS (1998) Neuronal correlates of visibility and invisibility in the primate visual system. Nat Neurosci 1:144–149. https://doi.org/10.1038/393
Mendoza JL (1980) A significance test for multisample sphericity. Psychometrika 45:495–498
Naish P (1980) The effects of graphemic and phonemic similarity between targets and masks in a backward visual masking paradigm. Q J Exp Psychol 32(1):57–68
Nelson SB (1991) Temporal interactions in the cat visual system. I. Orientation-selective suppression in the visual cortex. J Neurosci 11(2):344–356
Noguchi Y, Kakigi R (2005) Neural mechanisms of visual backward masking revealed by high temporal resolution imaging of human brain. Neuroimage 27(1):178–187
Ono F, Kitazawa S (2010) Shortening of subjective tone intervals followed by repetitive tone stimuli. Atten Percept Psychophys 72(2):492–500
Ono F, Kitazawa S (2011) Shortening of subjective visual intervals followed by repetitive stimulation. PLoS ONE 6(12):e28722
Ortega L, Guzman-Martinez E, Grabowecky M, Suzuki S (2012) Flicker adaptation of low-level cortical visual neurons contributes to temporal dilation. J Exp Psychol Hum Percept Perform 38(6):1380–1389. https://doi.org/10.1037/a0029495
Oyama T, Watanabe T, Funakawa M (1983) Effects of test-mask similarity on forward and backward masking of patterns by patterns. Psychol Res 45(3):303–313
Pariyadath V, Eagleman DM (2007) The effect of predictability on subjective duration. PLoS ONE 2(11):e1264
Pariyadath V, Eagleman DM (2012) Subjective duration distortions mirror neural repetition suppression. PLoS ONE 7(12):e49362
Peterson DJ, Gözenman F, Arciniega H, Berryhill ME (2015) Contralateral delay activity tracks the influence of Gestalt grouping principles on active visual working memory representations. Atten Percept Psychophys 77(7):2270–2283
Quinlan PT, Wilton RN (1998) Grouping by proximity or similarity? Competition between the Gestalt principles in vision. Perception 27(4):417–430
Raftery AE (1995) Bayesian model selection in social research. In: Marsden PV (ed) Sociological methodology 1995. Blackwell, Cambridge, pp 111–196
Sadibolova R, Sun S, Terhune DB (2021) Using adaptive psychophysics to identify the neural network reset time in subsecond interval timing. Exp Brain Res 239(12):3565–3572
Sekuler RW (1965) Spatial and temporal determinants of visual backward masking. J Exp Psychol 70(4):401–406
Shaffer JP (1986) Modified sequentially rejective multiple test procedures. J Am Stat Assoc 81(395):826–831
Spencer RM, Karmarkar U, Ivry RB (2009) Evaluating dedicated and intrinsic models of temporal encoding by varying context. Philos Trans R Soc b: Biol Sci 364(1525):1853–1863
Villalba-García C, Santaniello G, Luna D, Montoro PR, Hinojosa JA (2018) Temporal brain dynamics of the competition between proximity and shape similarity grouping cues in vision. Neuropsychologia 121:88–97
Wagemans J, Elder JH, Kubovy M, Palmer SE, Peterson MA, Singh M, von der Heydt R (2012) A century of Gestalt psychology in visual perception: I. Perceptual grouping and figure–ground organization. Psychol Bull 138(6):1172–1217
Wearden JH, Edwards H, Fakhri M, Percival A (1998) Why “sounds are judged longer than lights”: application of a model of the internal clock in humans. Q J Exp Psychol 51(2):97–120
Zhou B, Qin J, Mao L, Han S, Pöppel E (2010) Modulations of temporal perception by consciously and unconsciously perceived stimuli. Perception 39(7):900–908
Zhou B, Yang S, Mao L, Han S (2014) Visual feature processing in the early visual cortex affects duration perception. J Exp Psychol Gen 143(5):1893–1902
Zhou B, Yang S, Zhang T, Zhang X, Mao L (2015) Situational context is important: perceptual grouping modulates temporal perception. Cogn Process 16(1):443–447
Zimmermann E, Born S, Fink GR, Cavanagh P (2014) Masking produces compression of space and time in the absence of eye movements. J Neurophysiol 112(12):3066–3076
Acknowledgements
I am grateful to all participants. I would like to thank Dr. Haruyuki Kojima and Dr. Makoto Ichikawa for their support. Further, I would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by JSPS KAKENHI (grant numbers: 18H05806, 19K20998, and 21J00537).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asaoka, R. Stimulus (dis)similarity can modify the effect of a task-irrelevant sandwiching stimulus on the perceived duration of brief visual stimuli. Exp Brain Res 241, 889–903 (2023). https://doi.org/10.1007/s00221-023-06564-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06564-2