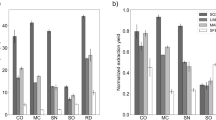
Abstract
A new process for enzyme-assisted extraction of polyphenols from de-aromatized rose (Rosa damascena Mill.) petals, primary by-product of essential oil production, was developed. Among the 19 major compounds analysed by liquid chromatography–mass spectrometry, 5 hydrolyzable tannins and 14 flavonols were detected in the rose petal extract. To the best of our knowledge, the presence of galloylquinic acid and ellagitannins has not been described before in Rosa damascena. The enzymatic processing led to 1.5–1.8 times higher contents of individual flavonols as compared to the control (without enzymatic treatment) sample. The co-pigmentation efficiency of enzymatically extracted rose petal polyphenols was evaluated regarding color stabilization in strawberry processing. The results obtained demonstrate that the addition (0.5%, w/w) of rose petal extract enhances the color intensity of strawberry spread, thus meeting the growing consumer demand for substitution of synthetic food additives by natural alternatives.




Similar content being viewed by others
Availability of data and materials
All authors make sure that all data and materials support their published claims and comply with standards.
Code availability
No software application or custom code used.
Abbreviations
- UHPLC-DAD:
-
Ultra high-performance liquid chromatography–diode array detection
- LC–MS:
-
Liquid chromatography–mass spectrometry
- TPP:
-
Total polyphenols
- TMA:
-
Total monomeric anthocyanins
- QGE:
-
Quercetin glucoside equivalent
- GAE:
-
Gallic acid equivalent
- CGE:
-
Cyanidin glucoside equivalent
- HHDP:
-
Hexahydroxydiphenoyl
References
Fenglin H, Ruili L, Bao H, Liang M (2004) Free radical scavenging activity of extracts prepared from fresh leaves of selected. Chin Med Plants Fitoterapia 75:14–23
Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC (2003) Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine 10:544–551
Ng TB, He JS, Niu SM, Zhao L, Pi ZF, Shao W, Liu F (2004) A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J Pharmacy Pharmacol 56:537–545
VanderJagt TJ, Ghattas R, VanderJagt DJ, Crossey M, Glew RH (2002) Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci 70:1035–1040
Vinokur Y, Rodov V, Reznick N, Goldman G, Horev B, Umiei N, Friedman H (2006) Rose petal tea as an antioxidant rich beverage cultivar effects. Food Sci 71:842–847
Choi EM, Hwang JK (2003) Investigations of anti-inflammatory and antinociceptive activities of Piper cubeba, Physalisangulata and Rosa hybrid. J Ethnopharmacol 89:171–175
Anesini C, Perez C (1993) Screening of plants used in Argentine folk medicine for antimicrobial activity. J Ethnopharmacol 39:119–128
Perez C, Anesini C (1994) In vitro antibacterial activity of Argentine folk medicinal plants against Salmonella typhi. J Ethnopharmacol 44:41–46
Mahmood N, Piacente S, Pizza C, Burke A, Khan AI, Hay AJ (1996) The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophys Res Commun 229:73–79
Dixit N, Tripathi C, Upadhyay R (1976) The antifungal substance of rose flowers (Rosa indica). Econ Bot 30:371–374
Kaul VK, Singh V, Singh B (2000) Damask rose and marigold: prospective industrial crops. J Med Aromatic Plant Sci 22:313–318
Kovats E (1987) Composition of essential oils. Part 7. Bulgarian oil of rose (Rosa damascena Mill.). J Chromatogr A 406:185–222
Umezu T, Ito H, Nagano K, Yamakoshi M, Oouchi H, Sakaniwa M, Morita M (2002) Anticonflict effects of rose oil and identification of its active constituents. Life Sci 72:91–102
Schieber A, Mihalev K, Berardini N, Mollov P, Carle R (2005) Flavonol glycosides from distilled petals of Rosa damascene Mill. Z Naturforsch 60:379–384
Pinelo-Jiménez M, Meyer AS (2008) Enzyme-assisted extraction of antioxidants: release of phenols from vegetal matrixes. Elec J Env Agricult Food Chem Title 7:3217–3220
Dinkova R, Heffels P, Shikov V, Weber F, Schieber A, Mihalev K (2014) Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vacciniummyrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Food Res Int 65:35–41
Kalcheva-Karadzhova K, Shikov V, Mihalev K, Dobrev G, Ludneva D, Penov N (2014) Enzyme-assisted extraction of polyphenols from rose (Rosa Damascena Mill.) Petals. Acta Universitatis Cibiniensis Series E: Food technology 18, no. 2
Clifford MN (2000) Anthocyanins—nature, occurrence and dietary burden. J Sci Food Agric 80:1063–1072
Hayashi K, Ohara N, Tsukui A (1996) Stability of anthocyanins in various vegetables and fruits. Food Sci Technol Int 2:30–33
Shikov V, Kammerer DR, Mihalev K, Mollov P, Carle R (2008) Heat stability of strawberry anthocyanins in model solutions containing natural copigments extracted from rose (Rosa damascena Mill.) petals. J Agric Food Chem 56:8521–8526
Mollov P, Mihalev K, Shikov V, Yoncheva N, Karagyozov V (2007) Colour stability improvement of strawberry beverage by fortification with polyphenolic copigments naturally occurring in rose petals. Innov Food Sci Emerg Technol 8:318–321
Baser KHC, Altintas A, Kurkcuoglu M (2012) Turkish Rose: a review of the history, ethnobotany and modern uses of rose petals, rose oil, rose water and other rose products. HerbalGram 96:40–53
Landbo AK, Meyer AS (2004) Effects of different enzymatic maceration treatments on enhancement of anthocyanins and other phenolics in black currant juice. Innov Food Sci Emerg Technol 5:503–513
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. John Wiley & Sons, New York, p F1.2.1-F1.2.13
Moyer AR, Hummer EK, Finn EC, Frei B, Wrolstad ER (2002) Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, and Ribes. J Agric Food Chem 50:519–525
Cai YZ, Xing J, Mei S, Zhan ZQ, Corke H (2005) Phenolic antioxidants (Hydrolyzable Tannins, Flavonols, and Anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J Agric Food Chem 53:9940–9948
Ishida Y, Kitagawa K, Goto K, Ohtani H (2005) Solid sampling technique for direct detection of condensed tannins in bark by matrix-assisted laser desorption/ ionization mass spectrometry. Rapid Commun Mass Spectrom 19:706–710
Cai YZ, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237
Feuereisen MM, Hoppe J, Zimmermann BF, Weber F, Schulze-Kaysers N, Schieber A (2014) Characterization of phenolic compounds in Brazilian pepper (Schinus terebinthifolius Raddi) exocarp. J Agric Food Chem 62:6219–6226
Cunja V, Mikulic-Petkovsek M, Stampar F, Schmitzer V (2014) Compound identification of selected rose species and cultivars: an insight to petal and leaf phenolic profiles. J Am Soc Hortic Sci 139:157–166
Meyers KJ, Swiecki TJ, Mitchell AE (2006) Understanding thenative Californian diet: identification of condensed and hydrolyzable tannins in Tanoak acorns (Lithocarpus densiflorus). J Agric Food Chem 54:7686–7691
Nayeshiro K, Eugster CH (1989) NotizüberEllagitannine und Flavonol glycosideaus Rosenblüten. Helv Chim Acta 72:985–992
Okuda T, Yoshida T, Hatano T, Iwasaki M, Kubo M, Orime T, Toshizaki M, Naruhashi N (1992) Hydrolysable tannins as chemotaxonomic markers in the Rosaceae. Phytochemistry 31:3091–3096
Sanchez Maldonado AF, Schieber A, Gänzle M (2015) Plant defense mechanisms and enzymatic transformation products and their potential applications in food preservation: advantages and limitations. Trends Food Sci Technol 46:49–59
Masuda T, Iritani K, Yonemori S, Oyama Y, Takeda Y (2001) Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci Biotechnol Biochem 65:1302–1309
Berke B, de Freitas V (2007) A colorimetric study of oenin copigmented by procyanidins. J Agric Food Chem 87:260–265
Shikov V, Kammerer DR, Mihalev K, Mollov P, Carle R (2012) Antioxidant capacity and colour stability of texture-improved canned strawberries as affected by the addition of rose (Rosa damascena Mill.) petal extracts. Food Res Int 46:552–556
Gonnet JF (1999) Colour effects of co-pigmentation of anthocyanins revisited—2. A colorimetric look at the solutions of cyanin co-pigmented by rutin using the CIELAB scale. Food Chem 43:337–394
Acknowledgements
We are grateful to Ecomaat Ltd. (Mirkovo,Bulgaria) for providing the distilled rose petals.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RD, VS and KM. The first draft of the manuscript was written by RD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Compliance with ethics requirements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dinkova, R., Vardakas, A., Dimitrova, E. et al. Valorization of rose (Rosa damascena Mill.) by-product: polyphenolic characterization and potential food application. Eur Food Res Technol 248, 2351–2358 (2022). https://doi.org/10.1007/s00217-022-04051-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04051-6