Abstract
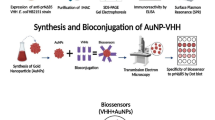
The harm and impact of the COVID-19 pandemic have highlighted the importance of fast, sensitive, and cost-effective virus detection methods. In this study, we developed a DNA aptamer sensor using nanoparticle-enhanced surface plasmon resonance imaging (SPRi) technology to achieve efficient labeling-free detection of SARS-CoV-2 S protein. We used the same DNA aptamer to modify the surface of the SPRi sensor chip and gold nanoparticles (AuNPs), respectively, for capturing target analytes and amplifying signals, achieving ideal results while greatly reducing costs and simplifying the preparation process. The SPRi sensing method exhibits a good linear relationship (R2 = 0.9926) in the concentration range of 1–20 nM before adding AuNPs to amplify the signal, with a limit of detection (LOD) of 0.32 nM. After amplifying the signal, there is a good linear relationship (R2 = 0.9829) between the concentration range of 25–1000 pM, with a LOD of 5.99 pM. The simulation results also verified the effectiveness of AuNPs in improving SPRi signal response. The SPRi sensor has the advantage of short detection time and can complete the detection within 10 min. In addition, the specificity and repeatability of this method can achieve excellent results. This is the first study to simultaneously capture a viral marker protein and amplify the signal using polyadenylic acid (polyA)–modified DNA aptamers on the SPR platform. This scheme can be used as a fast and inexpensive detection method for diagnosis at the point of care (POC) to combat current and future epidemics caused by the virus.







Similar content being viewed by others
References
Morales-Narvaez E, Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens Bioelectron. 2020;163:112274. https://doi.org/10.1016/j.bios.2020.112274.
Orooji Y, Sohrabi H, Hemmat N, Oroojalian F, Baradaran B, Mokhtarzadeh A, et al. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nanomicro Lett. 2021;13(1):18. https://doi.org/10.1007/s40820-020-00533-y.
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. https://doi.org/10.1038/s41467-020-15562-9.
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-92 e6. https://doi.org/10.1016/j.cell.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-80 e8. https://doi.org/10.1016/j.cell.2020.02.052.
Ji T, Liu Z, Wang G, Guo X, Akbar Khan S, Lai C, et al. Detection of COVID-19: a review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455. https://doi.org/10.1016/j.bios.2020.112455.
Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists? Eur Radiol. 2020;30(6):3266–7. https://doi.org/10.1007/s00330-020-06748-2.
Suleman S, Shukla SK, Malhotra N, Bukkitgar SD, Shetti NP, Pilloton R, et al. Point of care detection of COVID-19: advancement in biosensing and diagnostic methods. Chem Eng J. 2021;414:128759. https://doi.org/10.1016/j.cej.2021.128759.
Conklin SE, Martin K, Manabe YC, Schmidt HA, Miller J, Keruly M, et al. Evaluation of serological SARS-CoV-2 lateral flow assays for rapid point-of-care testing. J Clin Microbiol. 2021;59(2). https://doi.org/10.1128/JCM.02020-20.
Kilic T, Weissleder R, Lee H. Molecular and immunological diagnostic tests of COVID-19: current status and challenges. iScience. 2020;23(8):101406. https://doi.org/10.1016/j.isci.2020.101406.
Wang C, Liu M, Wang Z, Li S, Deng Y, He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today. 2021;37:101092. https://doi.org/10.1016/j.nantod.2021.101092.
Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–42. https://doi.org/10.1021/acsnano.0c02823.
Yang Y, Peng Y, Lin C, Long L, Hu J, He J, et al. Human ACE2-functionalized gold “virus-trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nanomicro Lett. 2021;13:109. https://doi.org/10.1007/s40820-021-00620-8.
Cennamo N, Pasquardini L, Arcadio F, Lunelli L, Vanzetti L, Carafa V, et al. SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor. Talanta. 2021;233:122532. https://doi.org/10.1016/j.talanta.2021.122532.
Yano TA, Kajisa T, Ono M, Miyasaka Y, Hasegawa Y, Saito A, et al. Ultrasensitive detection of SARS-CoV-2 nucleocapsid protein using large gold nanoparticle-enhanced surface plasmon resonance. Sci Rep. 2022;12(1):1060. https://doi.org/10.1038/s41598-022-05036-x.
Maria MSM. Surface plasmon resonance applications in clinical analysis. Anal Bioanal Chem. 2014;406(9–10):2303–23. https://doi.org/10.1007/s00216-014-7647-5.
Takemura K. Surface plasmon resonance (SPR)- and localized SPR (LSPR)-based virus sensing systems: optical vibration of nano- and micro-metallic materials for the development of next-generation virus detection technology. Biosensors (Basel). 2021;11(8). https://doi.org/10.3390/bios11080250.
Qu JH, Leirs K, Maes W, Imbrechts M, Callewaert N, Lagrou K, et al. Innovative FO-SPR label-free strategy for detecting anti-RBD antibodies in COVID-19 patient serum and whole blood. ACS Sens. 2022;7(2):477–87. https://doi.org/10.1021/acssensors.1c02215.
Huang L, Ding L, Zhou J, Chen S, Chen F, Zhao C, et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens Bioelectron. 2021;171:112685. https://doi.org/10.1016/j.bios.2020.112685.
Dehghani S, Nosrati R, Yousefi M, Nezami A, Soltani F, Taghdisi SM, et al. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): a review. Biosens Bioelectron. 2018;110:23–37. https://doi.org/10.1016/j.bios.2018.03.037.
Ulrich H. RNA aptamers: from basic science towards therapy. Handb Exp Pharmacol. 2006;173:305–26. https://doi.org/10.1007/3-540-27262-3_15.
Bouchard PR, Hutabarat RM, Thompson KM. Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol. 2010;50:237–57. https://doi.org/10.1146/annurev.pharmtox.010909.105547.
Liu B, Liu J. Freezing directed construction of bio/nano interfaces: reagentless conjugation, denser spherical nucleic acids, and better nanoflares. J Am Chem Soc. 2017;139(28):9471–4. https://doi.org/10.1021/jacs.7b04885.
Pei H, Li F, Wan Y, Wei M, Liu H, Su Y, et al. Designed diblock oligonucleotide for the synthesis of spatially isolated and highly hybridizable functionalization of DNA-gold nanoparticle nanoconjugates. J Am Chem Soc. 2012;134(29):11876–9. https://doi.org/10.1021/ja304118z.
Huertas CS, Soler M, Estevez MC, Lechuga LM. One-step immobilization of antibodies and DNA on gold sensor surfaces via a poly-adenine oligonucleotide approach. Anal Chem. 2020;92(18):12596–604. https://doi.org/10.1021/acs.analchem.0c02619.
Schreiner SM, Shudy DF, Hatch AL, Opdahl A, Whitman LJ, Petrovykh DY. Controlled and efficient hybridization achieved with DNA probes immobilized solely through preferential DNA-substrate interactions. Anal Chem. 2010;82(7):2803–10. https://doi.org/10.1021/ac902765g.
Zhang Z, Li J, Gu J, Amini R, Stacey HD, Ang JC, et al. A universal DNA aptamer that recognizes spike proteins of diverse SARS-CoV-2 variants of concern. Chemistry. 2022;28(15):e202200078. https://doi.org/10.1002/chem.202200078.
Hu M, Yuan C, Tian T, Wang X, Sun J, Xiong E, et al. Single-step, salt-aging-free, and thiol-free freezing construction of AuNP-based bioprobes for advancing CRISPR-based diagnostics. J Am Chem Soc. 2020;142(16):7506–13. https://doi.org/10.1021/jacs.0c00217.
Zhu D, Yan Y, Lei P, Shen B, Cheng W, Ju H, et al. A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA-AuNPs probe. Anal Chim Acta. 2014;846:44–50. https://doi.org/10.1016/j.aca.2014.07.024.
Fang Y, Jiang L, Jin S, Li Y, Jiang C, Zhang X, et al. AuNPs beacons-enhanced surface plasmon resonance imaging sensor for rapid, high-throughput and ultra-sensitive detection of three fusion genes related to acute promyelocytic leukemia. Sens Actuators B Chem. 2022;361. https://doi.org/10.1016/j.snb.2022.131728.
Li Y, Zou Y, Tan H, Jiang L, Fang Y, Jin S. Simultaneous and sensitive detection of two pathogenic genes of thrombotic diseases using SPRi sensor with one-step fixation probe by a poly-adenine oligonucleotide approach. Colloids Surf B Biointerfaces. 2022;209(Pt 1):112184. https://doi.org/10.1016/j.colsurfb.2021.112184.
Qian H, Huang Y, Duan X, Wei X, Fan Y, Gan D, et al. Fiber optic surface plasmon resonance biosensor for detection of PDGF-BB in serum based on self-assembled aptamer and antifouling peptide monolayer. Biosens Bioelectron. 2019;140:111350. https://doi.org/10.1016/j.bios.2019.111350.
Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans A Math Phys Eng Sci. 1915;2010(368):1333–83. https://doi.org/10.1098/rsta.2009.0273.
Hong X, Hall EA. Contribution of gold nanoparticles to the signal amplification in surface plasmon resonance. Analyst. 2012;137(20):4712–9. https://doi.org/10.1039/c2an35742a.
Sun M, Liu S, Song T, Chen F, Zhang J, Huang JA, et al. Spherical neutralizing aptamer inhibits SARS-CoV-2 infection and suppresses mutational escape. J Am Chem Soc. 2021;143(51):21541–8. https://doi.org/10.1021/jacs.1c08226.
Zeng S, Baillargeat D, Ho HP, Yong KT. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem Soc Rev. 2014;43(10):3426–52. https://doi.org/10.1039/c3cs60479a.
Ghosh SN. Electromagnetic theory and wave propagation. CRC Press. 2002.
Koledintseva MY, Dubroff RE, Schwartz RW. A Maxwell Garnett model for dielectric mixtures containing conducting particles at optical frequencies. Prog Electromagn Res. 2006;63:223–42. https://doi.org/10.2528/PIER06052601.
Ishimaru A. Electromagnetic wave propagation, radiation, and scattering: from fundamentals to applications. John Wiley & Sons. 2017.
Uchiho Y, Shimojo M, Furuya K, Kajikawa K. Optical response of gold-nanoparticle-amplified surface plasmon resonance spectroscopy. J Phys Chem C. 2010;114(11):4816–24. https://doi.org/10.1021/jp910438q.
Golden MS, Bjonnes AC, Georgiadis RM. Distance- and wavelength-dependent dielectric function of Au nanoparticles by angle-resolved surface plasmon resonance imaging. J Phys Chem C. 2010;114(19):8837–43. https://doi.org/10.1021/jp101850d.
Amouzadeh Tabrizi M, Nazari L, Acedo P. A photo-electrochemical aptasensor for the determination of severe acute respiratory syndrome coronavirus 2 receptor-binding domain by using graphitic carbon nitride-cadmium sulfide quantum dots nanocomposite. Sens Actuators B Chem. 2021;345:130377. https://doi.org/10.1016/j.snb.2021.130377.
Wang X, Zeng Y, Zhou J, Chen J, Miyan R, Zhang H, et al. Ultrafast surface plasmon resonance imaging sensor via the high-precision four-parameter-based spectral curve readjusting method. Anal Chem. 2021;93(2):828–33. https://doi.org/10.1021/acs.analchem.0c03347.
Subramanian P, Lesniewski A, Kaminska I, Vlandas A, Vasilescu A, Niedziolka-Jonsson J, et al. Lysozyme detection on aptamer functionalized graphene-coated SPR interfaces. Biosens Bioelectron. 2013;50:239–43. https://doi.org/10.1016/j.bios.2013.06.026.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province under Grant No. LZ22F050004 and the National Natural Science Foundation of China under Grant Nos. 52271139, 51972292, and 22204154.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, R., Zhou, Y., Fang, Y. et al. DNA aptamer-linked sandwich structure enhanced SPRi sensor for rapid, sensitive, and quantitative detection of SARS-CoV-2 spike protein. Anal Bioanal Chem 416, 1667–1677 (2024). https://doi.org/10.1007/s00216-024-05172-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-024-05172-5