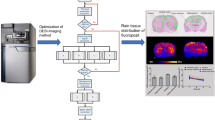
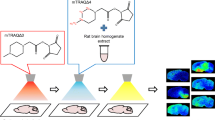
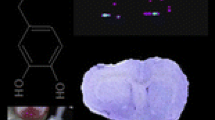
Abstract
Uncaria species (Rubiaceae) are used as traditional Chinese medicines (TCMs) to treat central nervous system (CNS) diseases, and monoterpene indole alkaloids are the main bioactive constituents. Localization and quantification of CNS drugs in fine brain regions are important to provide insights into their pharmacodynamics, for which quantitative mass spectrometry imaging (MSI) has emerged as a powerful technique. A systematic study of the quantitative imaging of seven Uncaria alkaloids in rat brains using desorption electrospray ionization mass spectrometry imaging (DESI-MSI) was presented. The distribution of the alkaloids in thirteen brain regions was quantified successfully using the calibration curves generated by a modified on-tissue approach. The distribution trend of different Uncaria alkaloids in the rat brain was listed as monoterpene indole alkaloids > monoterpene oxindole alkaloids, R-configuration epimers > S-configuration epimers. Particularly, Uncaria alkaloids were detected directly in the pineal gland for the first time and their enrichment phenomenon in this region had an instructive significance in future pharmacodynamic studies.
Graphical abstract






Similar content being viewed by others
References
Hsieh CL, Chen MF, Li TC, Li SC, Tang NY, Hsieh CT, et al. Anticonvulsant effect of Uncaria rhynchophylla (Miq) Jack. in rats with kainic acid-induced epileptic seizure. Am J Chin Med. 1999;27(2):257–64.
Huang H, Zhong R, Xia Z, Song J, Feng L. Neuroprotective effects of rhynchophylline against ischemic brain injury via regulation of the Akt/mTOR and TLRs signaling pathways. Molecules. 2014;19(8):11196–210.
Watanabe H, Zhao Q, Matsumoto K, Tohda M, Murakami Y, Zhang SH, et al. Pharmacological evidence for antidementia effect of Choto-san (Gouteng-san), a traditional Kampo medicine. Pharmacol Biochem Behav. 2003;75(3):635–43.
Niu Y, Li F, Inada C, Tanaka K, Watanabe S, Fujiwara H, et al. Chemical profiling with HPLC-FTMS of exogenous and endogenous chemicals susceptible to the administration of chotosan in an animal model of type 2 diabetes-induced dementia. J Pharm Biomed Anal. 2015;104:21–30.
Shao H, Yang Y, Mi Z, Zhu GX, Qi AP, Ji WG, et al. Anticonvulsant effect of rhynchophylline involved in the inhibition of persistent sodium current and NMDA receptor current in the pilocarpine rat model of temporal lobe epilepsy. Neuroscience. 2016;337:355–69.
Ho TY, Tang NY, Hsiang CY, Hsieh CL. Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1β and brain-derived neurotrophic factor. Phytomedicine. 2014;21(6):893–900.
Zhu W, Zhang Y, Huang Y, Lu L. Chinese herbal medicine for the treatment of drug addiction. Int Rev Neurobiol. 2017;135:279–95.
Fujiwara H, Iwasaki K, Furukawa K, Seki T, He M, Maruyama M, et al. Uncaria rhynchophylla, a Chinese medicinal herb, has potent antiaggregation effects on Alzheimer’s beta-amyloid proteins. J Neurosci Res. 2006;84(2):427–33.
Li HQ, Ip SP, Yuan QJ, Zheng GQ, Tsim KKW, Dong TTX, et al. Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun. 2019;82:264–78.
Xian YF, Mao QQ, Wu JC, Su ZR, Chen JN, Lai XP, et al. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. Journal of Alzheimer’s disease: JAD. 2014;39(2):331–46.
Qiao YL, Zhou JJ, Liang JH, Deng XP, Zhang ZJ, Huang HL, et al. Uncaria rhynchophylla ameliorates unpredictable chronic mild stress-induced depression in mice via activating 5-HT(1A) receptor: insights from transcriptomics. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2021;81: 153436.
Ikarashi Y, Sekiguchi K, Mizoguchi K. Serotonin receptor binding characteristics of geissoschizine methyl ether, an indole alkaloid in Uncaria hook. Curr Med Chem. 2018;25(9):1036–45.
Orlando G, Chiavaroli A, Leone S, Brunetti L, Politi M, Menghini L, et al. Inhibitory effects induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra water extracts on oxidative stress biomarkers and dopamine turnover in HypoE22 cells and isolated rat striatum challenged with 6-hydroxydopamine. Antioxidants (Basel). 2019;8(12):602.
Zheng M, Chen M, Liu C, Fan Y, Shi D. Alkaloids extracted from Uncaria rhynchophylla demonstrate neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the PI3K/Akt/mTOR signaling pathway. J Ethnopharmacol. 2021;266: 113451.
Gao L, Zhang Z, Feng Z, Wei W, Wu W, Zhi H, et al. Fast determination of 16 circulating neurotransmitters and their metabolites in plasma samples of spontaneously hypertensive rats intervened with five different Uncaria. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1179: 122856.
Zhang Q, Zhao JJ, Xu J, Feng F, Qu W. Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. J Ethnopharmacol. 2015;173:48–80.
Yang W, Ip SP, Liu L, Xian YF, Lin ZX. Uncaria rhynchophylla and its major constituents on central nervous system: a review on their pharmacological actions. Curr Vasc Pharmacol. 2020;18(4):346–57.
Hou J, Feng R, Zhang Y, Pan H, Yao S, Han S, et al. Characteristic chromatogram: a method of discriminate and quantitative analysis for quality evaluation of Uncaria stem with hooks. Planta Med. 2018;84(6–07):449–56.
Zhang YN, Yang YF, Xu W, Yang XW. The blood-brain barrier permeability of six indole alkaloids from Uncariae Ramulus Cum Uncis in the MDCK-pHaMDR cell monolayer model. Molecules. 2017;22(11):1944.
Kushida H, Fukutake M, Tabuchi M, Katsuhara T, Nishimura H, Ikarashi Y, et al. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria hook in rat plasma and brain after oral administration of the traditional Japanese medicine Yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed Chromatogr. 2013;27(12):1647–56.
Zhou Q, Ma J, Chen L. Tissue distribution of hirsutine and hirsuteine in mice by ultrahigh-performance liquid chromatography-mass spectrometry. J Anal Methods Chem. 2020;2020:7204315.
Wang W, Ma CM, Hattori M. Metabolism and pharmacokinetics of rhynchophylline in rats. Biol Pharm Bull. 2010;33(4):669–76.
Zhang C, Wu X, Xian Y, Zhu L, Lin G, Lin ZX. Evidence on integrating pharmacokinetics to find truly therapeutic agent for Alzheimer’s disease: comparative pharmacokinetics and disposition kinetics profiles of stereoisomers isorhynchophylline and rhynchophylline in rats. Evid Based Complement Alternat Med. 2019;2019:4016323.
Wang W, Luo S, Chen Y, Li B, Hattori M. Effective separation and simultaneous determination of corynoxeine and its metabolites in rats by high-performance liquid chromatography with tandem mass spectrometry and application to pharmacokinetics and in vivo distribution in main organs. Anal Sci. 2016;32(6):705–7.
Wang J, Qi P, Hou J, Shen Y, Yang M, Bi Q, et al. The profiling of the metabolites of hirsutine in rat by ultra-high performance liquid chromatography coupled with linear ion trap Orbitrap mass spectrometry: an improved strategy for the systematic screening and identification of metabolites in multi-samples in vivo. J Pharm Biomed Anal. 2017;134:149–57.
Tian X, Xu Z, Chen M, Hu P, Liu F, Sun Z, et al. Simultaneous determination of eight bioactive compounds by LC-MS/MS and its application to the pharmacokinetics, liver first-pass effect, liver and brain distribution of orally administrated Gouteng-Baitouweng (GB) in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1084:122–31.
Bourasset F, Auvity S, Thorne RG, Scherrmann JM. Brain distribution of drugs: brain morphology, delivery routes, and species differences. In: Handbook of experimental pharmacology. Berlin, Heidelberg: Springer; 2021. pp. 1–24.
Ntshangase S, Mdanda S, Naicker T, Kruger HG, Baijnath S, Govender T. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: application of matrix-assisted laser desorption/ionization mass spectrometry imaging and liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry : RCM. 2019;33(21):1643–51.
Buchberger AR, DeLaney K, Johnson J, Li L. Mass spectrometry imaging: a review of emerging advancements and future insights. Anal Chem. 2018;90(1):240–65.
Trim PJ, Snel MF. Small molecule MALDI MS imaging: current technologies and future challenges. Methods (San Diego, Calif). 2016;104:127–41.
Lietz CB, Gemperline E, Li L. Qualitative and quantitative mass spectrometry imaging of drugs and metabolites. Adv Drug Deliv Rev. 2013;65(8):1074–85.
Prideaux B, Stoeckli M. Mass spectrometry imaging for drug distribution studies. J Proteomics. 2012;75(16):4999–5013.
Shariatgorji M, Strittmatter N, Nilsson A, Källback P, Alvarsson A, Zhang X, et al. Simultaneous imaging of multiple neurotransmitters and neuroactive substances in the brain by desorption electrospray ionization mass spectrometry. Neuroimage. 2016;136:129–38.
Matsumoto T, Ikarashi Y, Takiyama M, Watanabe J, Setou M. Brain distribution of geissoschizine methyl ether in rats using mass spectrometry imaging analysis. Sci Rep. 2020;10(1):7293.
Tobias F, Hummon AB. Considerations for MALDI-based quantitative mass spectrometry imaging studies. J Proteome Res. 2020;19(9):3620–30.
Unsihuay D, Mesa Sanchez D, Laskin J. Quantitative mass spectrometry imaging of biological systems. Annu Rev Phys Chem. 2021;72:307–29.
Bo M. Rhynchophylline in rat blood by high-performance liquid chromatography-coupled microdialysis. J Med Plants Res. 2012;6(15):2977–84.
Wang W, Ma CM, Hattori M. Metabolism of isorhynchophylline in rats detected by LC-MS. J Pharm Pharm Sci: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2010;13(1):27–37.
Imamura S, Tabuchi M, Kushida H, Nishi A, Kanno H, Yamaguchi T, et al. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol. 2011;31(5):787–93.
O’Brien JS, Fillerup DL, Mead JF. Brain lipids: I. Quantification and fatty acid composition of cerebroside sulfate in human cerebral gray and white matter. J Lipid Res. 1964;5:109–16.
O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6(4):537–44.
Rzagalinski I, Kovačević B, Hainz N, Meier C, Tschernig T, Volmer DA. Toward higher sensitivity in quantitative MALDI imaging mass spectrometry of CNS drugs using a nonpolar matrix. Anal Chem. 2018;90(21):12592–600.
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res. 2020;69(2): e12667.
Song J. Pineal gland dysfunction in Alzheimer’s disease: relationship with the immune-pineal axis, sleep disturbance, and neurogenesis. Mol Neurodegener. 2019;14(1):28.
Yoo JH, Ha T-W, Hong JT, Oh K-W. Rhynchophylline, one of major constituents of Uncariae Ramulus et Uncus enhances pentobarbital-induced sleep behaviors and rapid eye movement sleep in rodents. Nps. 2016;22(4):263–9.
Shi JS, Huang B, Wu Q, Ren RX, Xie XL. Effects of rhynchophylline on motor activity of mice and serotonin and dopamine in rat brain. Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1993;14(2):114–7.
Sakakibara I, Terabayashi S, Kubo M, Higuchi M, Komatsu Y, Okada M, et al. Effect on locomotion of indole alkaloids from the hooks of uncaria plants. Phytomedicine : international journal of phytotherapy and phytopharmacology. 1999;6(3):163–8.
Quílez AM, Saenz MT, García MD. Uncaria tomentosa (Willd. ex. Roem. & Schult.) DC. and Eucalyptus globulus Labill. interactions when administered with diazepam. Phytotherapy research: PTR. 2012;26(3):458–61.
Sun Y-z, Liu R. Therapeutic evaluation on needling method of regulating the conception vessel and calming the mind for perimenopausal sleep disorder. J Acupunct Tuina Sci. 2013;11(3):142–6.
Pan W, Kwak S, Li G, Chen Y, Cai D. Therapeutic effect of Yang-Xue-Qing-Nao granules on sleep dysfunction in Parkinson’s disease. Chin Med. 2013;8:14.
Aizawa R, Kanbayashi T, Saito Y, Ogawa Y, Sugiyama T, Kitajima T, et al. Effects of Yoku-kan-san-ka-chimpi-hange on the sleep of normal healthy adult subjects. Psychiatry Clin Neurosci. 2002;56(3):303–4.
Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids and barriers of the CNS. 2011;8(1):7.
Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, et al. Evaluation of the protective effects of alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J Pharm Pharmacol. 1999;51(6):715–22.
Wu W, Zhang Z, Li F, Deng Y, Lei M, Long H, et al. A network-based approach to explore the mechanisms of Uncaria alkaloids in treating hypertension and alleviating Alzheimer’s disease. Int J Mol Sci. 2020;21(5):1766.
Acknowledgements
We thank Ms. Jing Huang in Waters Corporation for cooperation support. We thank Wei Rao, Emmanuelle Claude, Jonathan P. Willams, Mark Towers, Philippa Hart, and Yanchao Shi in Waters Corporation for the technical support. We also thank Professor Emeritus Geoffrey A. Cordell for editorial contributions in reviewing the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82003915 and 82003939).
Author information
Authors and Affiliations
Contributions
Lei Gao: conceptualization, methodology, software, writing—original draft, data curation, validation, formal analysis, investigation, visualization. Zijia Zhang: conceptualization, methodology, visualization, resources, writing—review and editing, funding acquisition. Wenyong Wu: methodology, software, validation. Yanping Deng: resources. Haijuan Zhi: resources, funding acquisition. Huali Long: resources. Min Lei: resources, validation. Jinjun Hou: conceptualization, methodology, software, validation, writing—review and editing, project administration, funding acquisition. Wanying Wu: resources, supervision, writing—review and editing, project administration, funding acquisition. De-an Guo: resources, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
The use of laboratory animals and all study protocols were approved by the Institutional Animal Care and Use Committee at the Chinese Academy of Sciences Shanghai Institute of Materia Medica.
Source of biological material
Male Wistar rats (200 ± 20 g) were purchased from the SLAC Lab Animal Center (Shanghai, China).
Statement on animal welfare
The management and use of animals during the experiment comply with the relevant regulations of “Guide for the Care and Use of Laboratory Animals issued by the National Research Council of the United States (2010),” “Regulations for the administration of affairs concerning laboratory animals (2017)” issued by the Science and Technology Commission of China, and the relevant regulations provided by the Institutional Animal Care and Use Committee at the Chinese Academy of Sciences Shanghai Institute of Materia Medica.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, L., Zhang, Z., Wu, W. et al. Quantitative imaging of natural products in fine brain regions using desorption electrospray ionization mass spectrometry imaging (DESI-MSI): Uncaria alkaloids as a case study. Anal Bioanal Chem 414, 4999–5007 (2022). https://doi.org/10.1007/s00216-022-04130-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04130-3