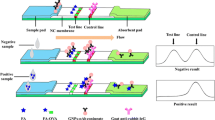
Abstract
Immunochromatographic assay (ICA) has been used widely for the onsite monitoring of illegal additives due to its simplicity, speed, and low cost. However, a scanner is commonly required for ICA to achieve quantitative results. In this work, we developed a visual semi-quantitative ICA for sibutramine, a banned additive in diet foods, without the need for a scanner for measurement. Monoclonal antibodies specific for sibutramine were raised and conjugated with upconversion nanoparticles (UCNPs) as the luminescent tracer. ICA was developed by employing multiple test lines to achieve the semi-quantitative detection of sibutramine. Based on the optimal conditions, the cutoff levels (limit of quantitation, LOQ) of T1 line, T2 line, T3 line, and T4 line were 0.02 μg/mL, 0.15 μg/mL, 1.0 μg/mL, and 7.5 μg/mL, respectively, in buffer system. The ICA demonstrated a LOQ at 0.2 mg/kg for sibutramine in diet food samples. The assay (including pretreatment) can be finished within 30 min without the aid of other instruments, except a laser pen. No false positive or false negative results were observed. The results indicated that the proposed method was reliable, simple, and rapid for the screening of sibutramine abuse in diet food samples.






Similar content being viewed by others
References
Connoley IP, et al. Thermogenic effects of sibutramine and its metabolites. Brit J Pharmacol. 1999;126:1487–95. https://doi.org/10.1038/sj.bjp.0702446.
Chen S, et al. Psychosis associated with usage of herbal slimming products adulterated with sibutramine, a case series. Clin Toxicol. 2010;48:832–8. https://doi.org/10.3109/15563650.2010.517208.
Stypułkowska K, et al. X-ray powder diffractometry and liquid chromatography studies of sibutramine and its analogues content in herbal dietary supplements. J Pharmaceut Biomed. 2011;56:969–75. https://doi.org/10.1016/j.jpba.2011.08.028.
Wilson P, Masse C. Detection of synthetic drugs as adulterants in natural and herbal slimming products by LC-ESI-MS/MS with polarity switching. J AOAC Int. 2016;99:929–40. https://doi.org/10.5740/jaoacint.15-0295.
Deconinck E, et al. A validated ultra high pressure liquid chromatographic method for the characterisation of confiscated illegal slimming products containing anorexics. J Pharmaceut Biomed. 2012;59:38–43. https://doi.org/10.1016/j.jpba.2011.09.036.
Bae JW, et al. Simultaneous determination of sibutramine and its active metabolites in human plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed Chromatogr. 2011;25:1181–8. https://doi.org/10.1002/bmc.1587.
Dunn JD, et al. Using a portable ion mobility spectrometer to screen dietary supplements for sibutramine. J Pharmaceut Biomed. 2011;54:469–74. https://doi.org/10.1016/j.jpba.2010.09.017.
Likar MD, et al. Rapid identification and absence of drug tests for AG-013736 in 1mg Axitinib tablets by ion mobility spectrometry and DART mass spectrometry. J Pharmaceut Biomed. 2011;55:569–73. https://doi.org/10.1016/j.jpba.2011.02.021.
Wang S, et al. Determination of sibutramine with a new sensor based on luminol electrochemiluminescence. J Lumin. 2011;131:1515–9. https://doi.org/10.1016/j.jlumin.2011.03.038.
de Carvalho LM, et al. Determination of synthetic pharmaceuticals in phytotherapeutics by capillary zone electrophoresis with contactless conductivity detection CZE-C4D. Microchem J. 2010;l96:114–9. https://doi.org/10.1016/j.microc.2010.02.006.
Lu F, et al. A new method for testing synthetic drugs adulterated in herbal medicines based on infrared spectroscopy. Anal Chim Acta. 2007;589:200–7. https://doi.org/10.1016/j.aca.2007.03.007.
Teradal NL, Narayan PS, Jaladappagari S. Electro-reduced graphene oxide film modified glassy carbon electrode as an electrochemical sensor for sibutramine. Anal Methods-UK. 2013;5:7090. https://doi.org/10.1039/c3ay41575a.
Xu C et al. (2008) Enzyme-linked immunologic detection method of sibutramine hydrochloride. Chinese intellectual property administration. https://patents.google.com/patent/CN101413946A/en. Accessed 15 Apr 2009
Suryoprabowo S, et al. Gold immunochromatographic assay for simultaneous detection of sibutramine and sildenafil in slimming tea and coffee. Sci China Mater. 2020;63:654–9. https://doi.org/10.1007/s40843-019-1228-2.
Zhou J, et al. Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef and swine urine. J Agric Food Chem. 2014;62:12061–6. https://doi.org/10.1021/jf5029416.
Xu D, et al. Rapid detection of Campylobacter jejuni using fluorescent microspheres as label for immunochromatographic strip test. Food Sci Biotechnol. 2013;22:585–91. https://doi.org/10.1007/s10068-013-0118-5.
Zhang X, et al. An ultra-sensitive monoclonal antibody-based fluorescent microsphere immunochromatographic test strip assay for detecting aflatoxin M1 in milk. Food Control. 2016;60:588–95. https://doi.org/10.1016/j.foodcont.2015.08.040.
Panferov VG, et al. Setting up the cut-off level of a sensitive barcode lateral flow assay with magnetic nanoparticles. Talanta. 2017;164:69–76. https://doi.org/10.1016/j.talanta.2016.11.025.
Serebrennikova KV, Samsonova JV, Osipov AP. A semi-quantitative rapid multi-range gradient lateral flow immunoassay for procalcitonin. Microchim Acta. 2019;18:423. https://doi.org/10.1007/s00604-019-3550-2.
Fu X, et al. Development of colloidal gold-based lateral flow immunoassay for rapid qualitative and semi-quantitative analysis of ustiloxins A and B in rice samples. Toxins. 2017;9:79–90. https://doi.org/10.3390/toxins9030079.
Chamarthi S, et al. Chairside quantitative immunochromatographic evaluation of salivary cotinine and its correlation with chronic periodontitis. J Indian Soc Periodontol. 2012;16:508–12. https://doi.org/10.4103/0972-124X.106888.
Hu G, et al. A novel and sensitive fluorescence immunoassay for the detection of fluoroquinolones in animal-derived foods using upconversion nanoparticles as labels. Anal Bioanal Chem. 2015;407:8487–96. https://doi.org/10.1007/s00216-015-8996-4.
Wang P, et al. Novel fabrication of immunochromatographic assay based on up conversion phosphors for sensitive detection of clenbuterol. Biosens Bioelectron. 2016;77:866–70. https://doi.org/10.1016/j.bios.2015.10.053.
Farka Z, et al. Nanoparticle-based immunochemical biosensors and assays, recent advances and challenges. Chem Rev. 2017;117:9973–10042. https://doi.org/10.1021/acs.chemrev.7b00037.
Zhang S, et al. High-specificity quantification method for almond-by-products, based on differential proteomic analysis. Food Chem. 2016;194:522–8. https://doi.org/10.1016/j.foodchem.2015.08.056.
Wang JP, Zhang SX, Shen JZ. Technical note, a monoclonal antibody-based immunoassay for determination of ractopamine in swine feeds. J Anim Sci. 2006;84:1248–51. https://doi.org/10.2527/2006.8451248x.
Zhang S, et al. Development of a competitive indirect ELISA for high-throughput screening of hydrocortisone in cosmetic sample. Food Agric Immunol. 2019;30:594–605. https://doi.org/10.1080/09540105.2019.1608162.
Zhang S, et al. Upconversion luminescence nanoparticles-based immunochromatographic assay for quantitative detection of triamcinolone acetonide in cosmetics. Spectrochim Acta A. 2019;214:302–8. https://doi.org/10.1016/j.saa.2019.02.053.
Kim HJ, et al. Monitoring of 29 weight loss compounds in foods and dietary supplements by LC-MS/MS. Food Addit Contam A. 2014;31:777–83. https://doi.org/10.1080/19440049.2014.888497.
Zhang S, et al. Development of an upconversion luminescence nanoparticles–based immunochromatographic assay for the rapid detection of dexamethasone in animal tissue. Food Anal Methods. 2019;12:752–60. https://doi.org/10.1007/s12161-018-01411-5.
Shen H, et al. A membrane-based fluorescence-quenching immunochromatographic sensor for the rapid detection of tetrodotoxin. Food Control. 2017;81:101–6. https://doi.org/10.1016/j.foodcont.2017.06.001.
Funding
This work was financially supported by the Key-Area Research and Development Program of Guangdong Province (2019B020211002), the Guangdong Special Support Program (2019TX05N052), and the Generic Technique Innovation Team Construction of Modern Agriculture of Guangdong Province (2019KJ130).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human subjects. All animal experiments that described in the present study were performed in adherence to South China Agricultural University animal experiment center guidelines and approved by Animal Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, SW., Sun, YY., Sun, YM. et al. Visual upconversion nanoparticle-based immunochromatographic assay for the semi-quantitative detection of sibutramine. Anal Bioanal Chem 412, 8135–8144 (2020). https://doi.org/10.1007/s00216-020-02944-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02944-7