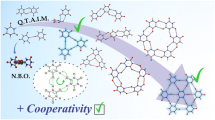
Abstract
Assembly of molecular systems into extended frameworks guided by weak non-covalent interactions is a hot topic of research in supramolecular chemistry. Herein, by an orchestration of intermolecular interactions guided by halogen bonding, we propose a computational designer strategy to steer the growth of graphyne-like molecular assemblies. Toward this, we analyze the halogen-bonded molecular assemblies of 1,3,5-triazine-based and benzene-1,3,5-tricarbonitrile-based monomers into graphyne-like and graphdiyne-like frameworks. The underlying halogen bonding interactions are quantified by way of intermolecular interaction energies and rationalized by way of molecular electrostatic potential and natural energy decomposition analysis, which allows for the separation of total intermolecular interaction energies into various components. The energetics of complexation indicate that, for the cyano-aromatic-based two-dimensional frameworks, iodo-substituted assemblies are stronger than the corresponding hydrogen-bonded assemblies and for the carbon nitride-based two-dimensional frameworks, bromo- and iodo-substituted assemblies are stronger than the hydrogen-bonded counterparts.











Similar content being viewed by others
References
Estarellas C, Bauzá A, Frontera A, Quiñonero D, Deyà PM (2011) Phys Chem Chem Phys 13:5696–5702
Bauzá A, Frontera A (2015) Angew Chem Int Ed 54:7340–7343
Parameswaran AM, James A, Aboobacker A, Srinivasamurthy Swathi R (2023) ChemPhysChem 24:e202200548
Murray JS, Shields ZPI, Seybold PG, Politzer P (2015) J Comput Sci 10:209–216
Colin M (1814) Ann Chim 91:252–272
Guthrie F (1863) J Chem Soc 16:239–244
Remsen I, Norris J (1896) Amer Chem J 18:90–95
Rhoussopoulos O (1883) Ber Dtsch Chem Ges 16:202–203
Seamon WH, Mallet JW (1881) Chem News 44:188–189
Benesi HA, Hildebrand J (1949) J Am Chem Soc 71:2703–2707
Benesi HA, Hildebrand JH (1948) J Am Chem Soc 70:2832–2833
Mulliken RS (1950) J Am Chem Soc 72:600–608
Bjorvatt T, Hassel O (1962) Acta Chem Scand 16:249–255
Hassel O, Rømming C (1962) Q Rev Chem Soc 16:1–18
Hassel O (1970) Science 170:497–502
Hassel O, Hvoslef J (1954) Acta Chem Scand 8:873
Olie K, Mijlhoff FC (1969) Acta Crystallogr B 25:974–977
Murray-Rust P, Motherwell WDS (1979) J Am Chem Soc 101:4374–4376
Ramasubbu N, Parthasarathy R, Murray-Rust P (1986) J Am Chem Soc 108:4308–4314
Bent HA (1968) Chem Rev 68:587–648
Murray JS, Paulsen K, Politzer P (1994) Proc Indian Acad Sci (Chem Sci) 106:267–275
Brinck T, Murray JS, Politzer P (1992) Int J Quantum Chem 44:57–64
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Legon AC (1999) Angew Chem Int Ed 38:2686–2714
Chopra D, Row TNG (2011) CrystEngComm 13:2175–2186
Lu Y-X, Zou J-W, Yu Q-S, Jiang Y-J, Zhao W-N (2007) Chem Phys Lett 449:6–10
Metrangolo P, Murray JS, Pilati T, Politzer P, Resnati G, Terraneo G (2011) Cryst Growth Des 11:4238–4246
Politzer P, Murray JS, Clark T (2015) In: Metrangolo P, Resnati G (eds) Halogen bonding I: impact on materials chemistry and life sciences pp. 19–42
Bauza A, Mooibroek TJ, Frontera A (2015) ChemPhysChem 16:2496–2517
Alkorta I, Elguero J, Frontera A (2020) Crystals 10:180
Murray JS, Politzer P (2020) Crystals 10:76
Metrangolo P, Resnati G (2001) Chem Eur J 7:2511–2519
Fourmigue M (2009) Curr Opin Solid State Mater Sci 13:36–45
Metrangolo P, Resnati G (2012) Cryst Growth Des 12:5835–5838
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) Chem Rev 116:2478–2601
Desiraju GR, Ho PS, Kloo L, Legon AC, Marquardt R, Metrangolo P, Politzer P, Resnati G, Rissanen K (2013) Pure Appl Chem 85:1711–1713
Xu M, Liang T, Shi M, Chen H (2013) Chem Rev 113:3766–3798
Niu T, Li A (2015) Prog Surf Sci 90:21–45
Owais C, James A, John C, Dhali R, Swathi RS (2018) J Phys Chem B 122:5127–5146
Yao F, Wang W, Shi H, Xu Z, Zeng M, Hu Y, Liu L, Ji X (2021) ACS Catal 11:14122–14147
James A, John C, Owais C, Myakala SN, Chandra Shekar S, Choudhuri JR, Swathi RS (2018) RSC Adv 8:22998–23018
Baughman RH, Eckhardt H, Kertesz M (1987) J Chem Phys 87:6687–6699
Insua I, Bergueiro J, Méndez-Ardoy A, Lostalé-Seijo I, Montenegro J (2022) Chem Sci 13:3057–3068
Slater AG, Beton PH, Champness NR (2011) Chem Sci 2:1440–1448
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM (2013) Science 341:1230444
Ding SY, Wang W (2013) Chem Soc Rev 42:548–568
Cote AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM (2005) Science 310:1166–1170
Teyssandier J, Mali KS, De Feyter S (2020) ChemistryOpen 9:225–241
Pang P, Li B, Zeng X, Miao X, Wang Y, Deng W (2021) J Phys Chem C 125:1378–1383
Yoon JK, Son W-j, Chung K-H, Kim H, Han S, Kahng S-J (2011) J Phys Chem C 115:2297–2301
Wang D, Lu X, Cai L, Zhang L, Feng S, Zhang W, Yang M, Wu J, Wang Z, Wee ATS (2022) ACS Nano 16:9843–9851
Silly F (2017) J Phys Chem C 121:10413–10418
Chung K-H, Park J, Kim KY, Yoon JK, Kim H, Han S, Kahng S-J (2011) Chem Commun 47:11492–11494
Mitchell JBO, Price SL, Leslie M, Buttar D, Roberts RJ (2001) J Phys Chem A 105:9961–9971
Yang Z, Fromm L, Sander T, Gebhardt J, Schaub TA, Görling A, Kivala M, Maier S (2020) Angew Chem Int Ed 59:9549–9555
Devore DP, Ellington TL, Shuford KL (2020) J Phys Chem A 124:10817–10825
Zhang S, Lu Y, Zhang Y, Peng C, Liu H (2017) J Phys Chem C 121:4451–4461
Singhal A, Kancharlapalli S, Ghosh SK (2018) J Mol Model 24:217
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456–1465
Weigend F (2006) Phys Chem Chem Phys 8:1057–1065
Hellweg A, Hättig C, Höfener S, Klopper W (2007) Theor Chem Acc 117:587–597
Riplinger C, Neese F (2013) J Chem Phys 138:034106
Riplinger C, Sandhoefer B, Hansen A, Neese F (2013) J Chem Phys 139:134101
Neese F (2012) WIREs Comput Mol Sci 2:73–78
Glendening ED, Streitwieser A (1994) J Chem Phys 100:2900–2909
Glendening ED, J KB, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Karafiloglou P, Landis CR, Weinhold F (2018). Theoretical Chemistry Institute, University of Wisconsin, Madison, Madison, WI
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016), Wallingford, CT
Li G, Li Y, Liu H, Guo Y, Li Y, Zhu D (2010) Chem Commun 46:3256–3258
Yang Z, Shen X, Wang N, He J, Li X, Wang X, Hou Z, Wang K, Gao J, Jiu T, Huang C (2019) ACS Appl Mater Interfaces 11:2608–2617
Burns LA, Marshall MS, Sherrill CD (2014) J Chem Theory Comput 10:49–57
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Badenhoop JK, Weinhold F (1997) J Chem Phys 107:5406–5421
Acknowledgements
The authors acknowledge the use of the Padmanabha cluster at the Centre for High-performance Computing at IISER TVM. R.S.S. acknowledges the Science and Engineering Research Board (SERB), Government of India for financial support of this work, through the SERB Core Research Grant (CRG/2022/006873). A.J. thanks IISER TVM for fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
This manuscript is dedicated to Prof. P. K. Chattaraj for his pivotal contributions to theoretical chemistry.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
James, A., Swathi, R.S. Halogen bonding: a designer strategy for graphyne-like two-dimensional architectures. Theor Chem Acc 142, 45 (2023). https://doi.org/10.1007/s00214-023-02987-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-02987-w