Abstract
Objective and methods
We investigated the locomotor, emotional, physiological, and neurobiological effects induced by low-dose reserpine repeated treatment (0.1 mg/kg; 14 injections) in males from the Lewis (LEW), Spontaneously Hypertensive Rats (SHR), and SHR.LEW-(D4Rat76-D4Mgh11) (SLA16) isogenic rat strains, which have different genetic backgrounds on chromosome 4. Behavioral responses in the catalepsy, open-field, and oral movements’ tests were coupled with blood pressure, body weight, and striatal tyrosine hydroxylase (TH) level assessments to establish neurobiological comparisons between reserpine-induced impairments and genetic backgrounds
Results
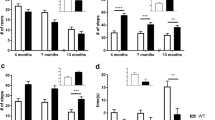
Results revealed the SHR strain was more sensitive in the catalepsy test and exhibited higher TH immunoreactivity in the dorsal striatum. The SLA16 strain presented more oral movements, suggesting increased susceptibility to develop oral dyskinesia.
Conclusions
Our results showed the efficacy of repeated treatment with a low dose of reserpine and demonstrated, for the first time, the genetic influence of a specific region of chromosome 4 on the expression of these effects.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Abílio VC, Silva RH, Carvalho RC, Grassl C, Calzavara MB, Registro S, D’almeida V, Ribeiro RA, Frussa-Frilho R (2004) Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology 47(2):263–272
Angrini M, Leslie JC, Shephard RA (1998) Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav 59:387–397
Anselmi M, Correa FJ, Santos JR, Silva AF, Cunha JA, Leão AH, Campêlo CL, Ribeiro AM, Silva RH, Izidio GS (2016) Genetic evidence for chromosome 4 loci influencing learning and memory. Neurobiol Learn Mem 131:182–191
Antkiewicz-Michaluk L, Wasik A, Mozdzen E, Romanska I, Michaluk J (2014) Antidepressant-like effect of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: behavioral and neurochemical studies in the rat. Neurotox Res 26(1):85–98
Bartels T, Choi JG, Selkoe DJ (2011) Properties of native brain α-synuclein. Nature 477:107–110
Bennett B (2000) Congenic strains developed for alcohol- and drug-related phenotypes. Pharmacol Biochem Behav 67(4):671–681
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington clinical, morphological and neurochemical correlations. J Neurol Sci 20(4):415–455
Bispo JMM, Melo JEC, Gois AM, Leal PC, Lins LCRF, Souza MF, Medeiros KAAL, Ribeiro AM, Silva RH, Marchioro M, Santos JR (2019) Sex differences in the progressive model of parkinsonism induced by reserpine in rats. Behav Brain Res 363:23–29
Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Campbell MC, Perlmutter JS, Kotzbauer PT (2015) Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol. 2(10):949–59
Campêlo CLC, Cagni FC, De Siqueira Figueredo D, Oliveira LG Jr, Silva-Neto AB, Macêdo PT, Santos JR, Izídio GS, Ribeiro AM, De Andrade TG, De Oliveira Godeiro CJR, Silva RH (2017) Variants in SNCA gene are associated with Parkinson’s disease risk and cognitive symptoms in a Brazilian sample. Front Aging Neurosci. 9:198
Chaudhry FA, Edwards RH, Fonnum F (2008) Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annu Rev Pharmacol Toxicol 48:277–301
Chiavegatto S, Izidio GS, Mendes-Lana A, Aneas I, Freitas TA, Torrão AS, Conceição IM, Britto LRG, Ramos A (2009) Expression of alpha-synuclein is increased in the hippocampus of rats with high levels of innate anxiety. Mol Psychiatry 14(9):894–905
Cicila GT, Morgan EE, Lee SJ, Farms P, Yerga-Woolwine S, Toland EJ, Ramdath RS, Gopalakrishnan K, Bohman K, Nestor-Kalinoski AL, Khuder SA, Joe B (2009) Epistatic genetic determinants of blood pressure and mortality in a salt-sensitive hypertension model. Hypertension (Dallas, Tex.: 1979) 53(4):725–732
Cintra RR, Lins LCRF, Medeiros KAAL, Souza MF, Gois AM, Bispo JMM, Melo MS, Leal PC, Meurer YSR, Ribeiro AM, Silva RH, Marchioro M, Santos JR (2021) Nociception alterations precede motor symptoms in a progressive model of parkinsonism induced by reserpine in middle-aged rats. Brain Res Bull 171:1–9
Corona JC, Duchen MR (2015) PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res 40(2):308–316
Corona JC, Duchen MR (2016) PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med. 100:153–163
De Medeiros GF, Pereira E, Granzotto N, Ramos A (2013) Low-anxiety rat phenotypes can be further reduced through genetic intervention. PLoS One 8(12):e83666
De Medeiros GF, Corrêa FJ, Corvino ME, Izidio GS, Ramos A (2014) The long way from complex phenotypes to genes: the story of rat chromosome 4 and its behavioral effects. World J Neurosci 4(3):203–215
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491
Dos Santos TFO, De R Santos E, Bispo JMM, De Souza MF, De Gois AM, Lins LCRF, Silva RH, Ribeiro AM, Marchioro M, Dos Santos JR (2021) Balance alterations and reduction of pedunculopontine cholinergic neurons in early stages of parkinsonism in middle-aged rats. Exp Gerontol 145:111198
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164(4):1357–1391
Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E (1996) Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A 93(10):5166–5171
Fernandes VS, Santos JR, Leão AH, Medeiros AM, Melo TG, Izídio GS, Cabral A, Ribeiro RA, Abílio VC, Ribeiro AM, Silva RH (2012) Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behav Brain Res 231(1):154–163
Gallegos S, Pacheco C, Peters C, Opazo CM, Aguayo LG (2015) Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson’s disease. Front Neurosci 9:59. eCollection 2015.
Gaugler MN, Genc O, Bobela W, Mohanna S, Ardah MT, El-Agnaf OM, Cantoni M, Bensadoun JC, Schneggenburguer R, Knott GW, Aebischer P, Schneider BL (2012) Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol 123(5):653–669
Hinojosa FR, Spricigo L, Izídio GS et al (2006) Evaluation of two genetic animal models in behavioral tests of anxiety and depression. Behav Brain Res 168(1):127–136
Jellinger KA (1991) Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14(3):153–197
Jenner P, Olanow CW (2006) The pathogenesis of cell death in Parkinson’s disease. Neurology 66:S24–S36
Klockgether T (2004) Parkinson’s disease: clinical aspects. Cell Tissue Res 318(1):115–120
Krüger R, Kuhn W, Müller T et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108
Labuda CJ, Fuchs PN (2002) Catecholamine depletion by reserpine blocks the anxiolytic actions of ethanol in the rat. Alcohol 26(1):55–59
Leão AH, Sarmento-Silva AJ, Santos JR, Ribeiro AM, Silva RH (2015) Molecular, neurochemical, and behavioral hallmarks of reserpine as a model for Parkinson’s disease: new perspectives to a long-standing model. Brain Pathol 25(4):377–390
Leão AH, Meurer YS, Da Silva AF, Medeiros AM, Campêlo CL, Abílio Vc, Engelberth RC, Cavalcante JS, Izídio GS, Ribeiro AM, Silva RH (2017) Spontaneously hypertensive rats (SHR) are resistant to a reserpine-induced progressive model of Parkinson’s disease: differences in motor behavior, tyrosine hydroxylase and α-synuclein expression. Front Aging Neurosci 9:78
Leão AH, Meurer YS, Freitas TA, Medeiros AM, Abílio VC, Izídio GS, Conceição IM, Ribeiro AM, Silva RH (2021) Changes in the mesocorticolimbic pathway after low dose reserpine-treatment in Wistar and spontaneously hypertensive rats (SHR): implications for cognitive deficits in a progressive animal model for Parkinson’s disease. Behav Brain Res 410:113349
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Li D, He L (2007) Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: a HuGE review and meta-analysis. Genet Med 9(1):4–8
Lima AC, Meurer YRS, Bioni VS, Cunha DMG, Gonçalves N, Lopes-Silva LB, Becegato M, Soares MBL, Marinho GF, Santos JR, Silva, RH (2021) Female Rats Are Resistant to Cognitive Motor and Dopaminergic Deficits in the Reserpine-Induced Progressive Model of Parkinson’s Disease Frontiers in Aging Neuroscience 13. https://doi.org/10.3389/fnagi.2021.757714
Masliah E, Rockstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269
Mazur FG, Oliveira LFG, Cunha MP, Rodrigues ALS, Ran P, Vendruscolo LF, Izídio GS (2017) Effects of physical exercise and social isolation on anxiety-related behaviors in two inbred rat strains. Behav Processes 142:70–78
Nalls MA, Plagnol V, Hernandez DG et al (2011) International Parkinson Disease Genomics Consortium. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377(9766):641–649
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. 6th Edition. Academic Press, San Diego
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJA (2002) role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090–3099
Queiroz CM, Piovezan RD, Frussa-Filho R (1998) Reserpine does not induce orofacial dyskinesia in spontaneously hypertensive rats. Eur J Pharmacol 356(2–3):105–108
Ramos A, Berton O, Mormede P, Chaouloff F (1997) A multiple-test study of anxiety-related behaviors in six inbred rat strains. Behav Brain Res 85(1):57–69
Ramos A, Mellerin Y, Mormède P, Chaouloff F (1998) A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav Brain Res 96(1–2):195–205
Ramos A, Moisan MP, Chaouloff F, Mormède C, Mormède P (1999) Identification of female-specific QTL affecting and emotionality-related behavior in rats. Mol Psychiatry 4(5):453–462
Ramos A, Granzotto N, Kremer R, Boeder AM, Araújo JFP, Pereira AG, Izídio GS (2022) Hunting for genes underlying emotionality in the laboratory rat: maps, tools and traps. Curr Neuropharmacol. Online ahead of print
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8(12):1128–1139
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102(5):748–759
Santos JR, Cunha JAS, Dierschnabel AL, Campêlo CL, Leão AH, Silva AF, Engelberth RC, Izídio GS, Cavalcante JS, Abílio VC, Ribeiro AM, Silva RH (2013) Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav Brain Res 253:68–77
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
SüdhoF TC, Starke K (2008) Pharmacology of neurotransmitter release. In: Handbook of Experimental Pharmacology, vol 184. Springer, p 83–87
Varghese F, Bukhari AB, Malhotra R, De A (2014) IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 9(5):e96801
Velázquez AM, Roversi K, Dillenburg-Pilla P, Rodrigues RF, Zárate-Bladés CR, Prediger RDS, Izídio GS (2019) The influence of chromosome 4 on metabolism and spatial memory in SHR and SLA16 rat strains. Behav Brain Res 370:111966
Wersinger C, Sidhu A (2003) Attenuation of dopamine transporter activity by α-synuclein. Neurosci Lett 340(3):189–192
Zaltieri M, Longhena F, Pizzi M, Missale C, Spano P, Bellucci A (2015) Mitochondrial dysfunction and α-synuclein synaptic pathology in Parkinson’s disease: who’s on first? Parkinson’s Disease 2015:ID 108029, 10p
Zarranz JJ, Alegre J, Gómez-Esteban JC et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–217
Acknowledgements
The authors would like to thank the “Laboratório Multiusuário de Estudos em Biologia” at the Federal University of Santa Catarina (LAMEB/UFSC) for technical support in processing samples for the immunohistochemistry assay.
Funding
This work was supported by Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) Grant No. 303325/2017–8, Edital MCT/CNPq 14/2010 and MCTI/CNPq 14/2013; and Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) Grant No. 2015/12308–5. G. P. Fadanni, N. Granzotto, and P.A.R dos Anjos were recipients of fellowships from CNPq. A.G. Pereira is supported by a scholarship from PNPD/CAPES.
Author information
Authors and Affiliations
Contributions
Guilherme Pasetto Fadanni participated in the research by doing experiments, analyzing data, and assisting in the writing of the manuscript. Anderson Henrique Leão participated in the research by doing experiments, analyzing data, and assisting in the writing of the manuscript. Natalli Granzotto participated in the research by doing experiments, analyzing data, and assisting in the writing of the manuscript. Aline Guimarães Pereira participated in the research by analyzing data and assisting in the writing of the manuscript. Auderlan Mendonça de Gois participated in the research by doing experiments, analyzing data, and assisting in the writing of the manuscript. Pâmela Andressa Ramborger Anjos participated in the research by doing experiments. Áurea Elizabeth Linder participated in the research by analyzing data and assisting in the writing of the manuscript. José Ronaldo Santos participated in the research by analyzing the data and assisting in the writing of the manuscript. Regina Helena Silva participated in the research by analyzing the data and assisting in the writing of the manuscript. Geison Souza Izídio participated in the research by analyzing the data and assisting in the writing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed following the guidelines for animal care from “Conselho Nacional de Controle de Experimentação Animal” (CONCEA, Brazil) and following the specifications of the local ethics committee (CEUA/UFSC, Protocol No. PP00903). The experimental protocol was designed to minimize the number of animals and procedures involving stress, pain, or discomfort.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Figure 1
Systolic blood pressure. Systolic blood pressure in Lewis (LEW), Spontaneously Hypertensive Rats (SHR) and SHR.LEW-(D4Rat76-D4Mgh11) isogenic rat strains (n = 8/strain/treatment) during repeated treatment with reserpine (RES, 0.1 mg/kg; 14 injections) or vehicle (VEH). Results are expressed as mean + S.E.M. ## = VEH>RES, p≤0.01; ** = SHR>LEW, p≤0.01 and SHR>SLA16, p≤0.05. Two-way ANOVA with repeated measures followed by Duncan's post hoc test (PNG 96 kb)

Supplementary Figure 2
Representation of chromosome 4 from SLA16 rats with positions of the molecular markers. The Differential Genomic Region (DGR) is represented in blue. The genetic background of SHR rats is represented in red. Some genes are mapped in the DGR region, for example, the Snca (alpha-synuclein), Grid2 (Glutamate Ionotropic Receptor Delta Type Subunit 2), Il12rb2 (Interleukin-12 receptor subunit beta-2), Tac1r (Tachykinin receptor 1), Grip2 (Glutamate Receptor Interacting Protein 2), Il5ra (Interleukin 5 receptor subunit alpha), Grm7 (Glutamate metabotropic receptor 7), Pparg (Peroxisome proliferator-activated receptor gamma), Grin2b (Glutamate ionotropic receptor NMDA type subunit 2B). (PNG 237 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fadanni, G.P., Leão, A.H.F.F., Granzotto, N. et al. Genetic effects in a progressive model of parkinsonism induced by reserpine. Psychopharmacology 240, 1131–1142 (2023). https://doi.org/10.1007/s00213-023-06350-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06350-6