Abstract
Rationale
Cannabidiol (CBD) is found in the cannabis plant and has garnered attention as a potential treatment for alcohol use disorder (AUD). CBD reduces alcohol consumption and other markers of alcohol dependence in rodents, but human research on CBD and alcohol is limited. It is unknown whether CBD reduces drinking in humans, and mechanisms through which CBD could impact behavioral AUD phenotypes are unknown.
Objectives
This study explores effects of oral CBD on breath alcohol level (BrAC), and subjective effects of alcohol in human participants who report heavy drinking.
Methods
In this placebo-controlled, crossover study, participants consumed 30 mg CBD, 200 mg CBD, or placebo CBD before receiving a standardized alcohol dose. Participants were blind to which CBD dose they received at each session and completed sessions in random order. Thirty-six individuals completed at least one session and were included in analyses.
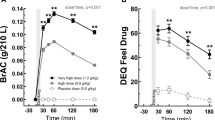
Results
Differences in outcomes across the three conditions and by sex were explored using multilevel structural equation models. BrAC fell fastest in the placebo condition, followed by 30 mg and 200 mg CBD. Stimulation decreased more slowly in the 200 mg CBD condition than in placebo (b = − 2.38, BCI [− 4.46, − .03]). Sedation decreased more slowly in the 30 mg CBD condition than in placebo (b = − 2.41, BCI [− 4.61, − .09]). However, the magnitude of condition differences in BrAC and subjective effects was trivial.
Conclusions
CBD has minimal influence on BrAC and subjective effects of alcohol. Further research is needed to test whether CBD impacts alcohol consumption in humans, and if so, what mechanism(s) may explain this effect.




Similar content being viewed by others
Data Availability
Data is available upon request to the corresponding author.
Notes
Note that the breathalyzer used in this study, the Intoximeter Alco-Sensor IV, takes measurements in grams of alcohol per 210L of breath, as is typical for breathalyzer devices used in the U.S. For simplicity, we report units of BrAC measurements throughout the paper as g/dL, as this is how BAC is typically reported, and it is consistent with how BrAC is reported in prior alcohol administration studies using BrAC measurements to approximate BAC (e.g., Bujarski et al. 2017).
Note that in the “Reasons for Ineligibility” box within Fig. 3, excluded individuals could have endorsed multiple reasons for ineligibility. For the purposes of reporting here, screened individuals were counted only once. If an individual did not meet cannabis use criteria, then “Did not meet cannabis use criteria” was listed as their reason for ineligibility, though it is possible that they also could have been excluded based on not meeting other criteria. If they met cannabis use criteria but did not meet alcohol use criteria, then “Did not meet alcohol use criteria” was listed as their reason for ineligibility, though it is possible that they also could have been excluded on the basis of additional ineligibility reasons listed below. The same rule applies to each successive reason for ineligibility listed in Fig. 3.
Note that BrAC models were run using BrAC*100 to aid in model convergence, but interpretations provided in text are transformed back to the typical scale (i.e., model estimates were divided by 100).
References
Beck AT, Steer RA, Carbin MG (1988) Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 8:77–100. https://doi.org/10.1016/0272-7358(88)90050-5
Beck AT, Steer RA (1990) Manual for the Beck Anxiety Inventory. Psychological Corporation, San Antonio, TX
Belgrave BE, Bird KD, Chesher GB et al (1979) The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacol 64:243–246
Bird KD, Boleyn T, Chesher GB et al (1980) Intercannabinoid and cannabinoid-ethanol interactions and their effects on human performance. Psychopharmacol 71:181–188. https://doi.org/10.1007/BF00434409
Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606
Brents LK (2016) Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med 89:175–191
Brick J (2006) Standardization of alcohol calculations in research. Alcohol Clin Exp Res 30:1276–1287
Brumback T, Cao D, King A (2007) Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend 91:10–17. https://doi.org/10.1016/J.DRUGALCDEP.2007.04.013
Bujarski S, Hutchison KE, Prause N, Ray LA (2017) Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict Biol 22:235–245
Consroe P, Carlini EA, Zwicker AP, Lacerda LA (1979) Interaction of cannabidiol and alcohol in humans. Psychopharmacol 66:45–50. https://doi.org/10.1007/BF00431988
Craft RM, Marusich JA, Wiley JL (2013) Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci 92(8–9):476–481
Dougherty DM, Marsh-Richard DM, Hatzis ES et al (2008) A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend 96:111–120. https://doi.org/10.1016/J.DRUGALCDEP.2008.02.002
Dutra L, Stathopoulou G, Basden SL et al (2008) A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psych 165:179–187. https://doi.org/10.1176/appi.ajp.2007.06111851
Ewell TR, Abbotts KSS, Williams NNB, Butterklee HM, Bomar MC, Harms KJ, ... Bell C (2021) Pharmacokinetic investigation of commercially available edible marijuana products in humans: potential influence of body composition and influence on glucose control. Pharmaceuticals 14(8):817. https://doi.org/10.3390/ph14080817
Filev R, Engelke DS, Da Silveira DX et al (2017) THC inhibits the expression of ethanol-induced locomotor sensitization in mice. Alcohol 65:31–35. https://doi.org/10.1016/j.alcohol.2017.06.004
Francés F, Portolés O, Castelló A et al (2015) Association between opioid receptor mu 1 (OPRM1) gene polymorphisms and tobacco and alcohol consumption in a Spanish population. Bosn J Basic Med Sci 15:31
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis, vol 3. CRC Texts in Statistical Science, Chapman & Hall
Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM et al (2018) Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacol 43:2036–2045. https://doi.org/10.1038/s41386-018-0050-8
Grant BF, Chou SP, Saha TD et al (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013. JAMA Psych 74:911. https://doi.org/10.1001/jamapsychiatry.2017.2161
Hendler RA, Ramchandani VA, Gilman J, Hommer DW (2013) Stimulant and sedative effects of alcohol. In: Sommer WH, Spanagel R (eds) Behavioral neurobiology of alcohol addiction. Springer, Berlin Heidelberg
Hindmarch I, Kerr JS, Sherwood N (1991) The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol Alcohol 26:71–79. https://doi.org/10.1093/oxfordjournals.alcalc.a045085
Jonas DE, Amick HR, Feltner C et al (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA 311:1889. https://doi.org/10.1001/jama.2014.3628
Karoly HC, Mueller RL, Bidwell LC, Hutchison KE (2020) Cannabinoids and the microbiota–Gut–Brain Axis: emerging effects of cannabidiol and potential applications to alcohol use disorders. Alcohol Clin Exp Res 44(2):340–353
King AC, De Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–399
Lappan SN, Brown AW, Hendricks PS (2020) Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction 115:201–217
Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108:172–182
Martin CS, Earleywine M, Musty RE et al (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–146
Mehta PD, Neale MC (2005) People are variables too: multilevel structural equations modeling. Psychol Methods 10:259
Morean ME, Corbin WR, Treat TA (2013) The subjective effects of alcohol scale: development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychol Assess 25:780
Musella A, Fresegna D, Rizzo FR et al (2017) A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci Rep 7:7363. https://doi.org/10.1038/s41598-017-07519-8
Muthén LK, Muthen B (2017) Mplus user’s guide: statistical analysis with latent variables, user’s guide. Muthén & Muthén, Los Angeles
Muthén B, Asparouhov T (2012) Bayesian structural equation modeling: a more flexible representation of substantive theory. Psychol Methods 17:313
Nona CN, Hendershot CS, Le Foll B (2019) Effects of cannabidiol on alcohol-related outcomes: Areview of preclinical and human research. Exp Clin Psychopharmacol. https://doi.org/10.1037/pha0000272
Parsons LH, Hurd YL (2015) Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16:579–594. https://doi.org/10.1038/nrn4004
Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS (2003) Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcohol Clin Exp Res 27:773–779
Posey D, Mozayani A (2007) The estimation of blood alcohol concentration. Forensic Sci Med Pathol 3:33–39
Preacher KJ, Zyphur MJ, Zhang Z (2010) A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods 15:209–233. https://doi.org/10.1037/a0020141
Preacher KJ, Zhang Z, Zyphur MJ (2016) Multilevel structural equation models for assessing moderation within and across levels of analysis. Psychol Methods 21:189–205. https://doi.org/10.1037/met0000052
Ramaekers JG, Theunissen EL, de Brouwer M et al (2011) Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacol 214:391–401. https://doi.org/10.1007/s00213-010-2042-1
Ray LA, Hutchison KE (2007) Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry 64:1069–1077
Ray LA, Hutchison KE, MacKillop J et al (2008) Effects of naltrexone during the descending limb of the blood alcohol curve. Am J Addict 17:257–264
Reinert DF, Allen JP (2007) The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 31:185–199
Rueger SY, Hu H, McNamara P et al (2015) Differences in subjective response to alcohol in heavy-and light-drinking Chinese men versus Caucasian American men. Addict 110:91–99
Sadler DW, Lennox S (2015) Intra-individual and inter-individual variation in breath alcohol pharmacokinetics: Variation over three visits. J Forensic Leg Med 34:88–98
Sadler DW, Parker J (2014) Intra-individual and inter-individual variation in breath alcohol pharmacokinetics: the effect of short-term variation. J Forensic Leg Med 25:77–84
Saunders JB, Aasland OG, Babor TF et al (1993) Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addict 88:791–804
Schacht JP, Voronin KE, Randall PK, Anton RF (2018) Dopaminergic genetic variation influences aripiprazole effects on alcohol self-administration and the neural response to alcohol cues in a randomized trial. Neuropsychopharmacol 43:1247–1256
Schlienz NJ, Budney AJ, Lee DC, Vandrey R (2017) Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep 4:75–81. https://doi.org/10.1007/s40429-017-0143-1
Sloan ME, Gowin JL, Ramchandani VA, Le Foll B (2017) The endocannabinoid system as a target for addiction treatment: trials and tribulations. Neuropharmacol 124:73–83. https://doi.org/10.1016/J.NEUROPHARM.2017.05.031
Taylor L, Gidal B, Blakey G et al (2018) A Phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32:1053–1067. https://doi.org/10.1007/s40263-018-0578-5
Team RC (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Turna J, Syan SK, Frey BN et al (2019) Cannabidiol as a novel candidate alcohol Use disorder pharmacotherapy: a systematic review. Alcohol Clin Exp Res 43(4):550–563. https://doi.org/10.1111/acer.13964
Viudez-Martínez A, García-Gutiérrez MS, Fraguas-Sánchez AI et al (2018a) Effects of cannabidiol plus naltrexone on motivation and ethanol consumption. Br J Pharmacol 175:3369–3378. https://doi.org/10.1111/bph.14380
Viudez-Martínez A, García-Gutiérrez MS, Navarrón CM et al (2018b) Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biol 23:154–164. https://doi.org/10.1111/adb.12495
Viudez-Martínez A, García-Gutiérrez MS, Manzanares J (2019) Gender differences in the effects of cannabidiol on ethanol binge drinking in mice. Addict Biol 25(3):12765. https://doi.org/10.1111/adb.12765
Wakley AA, Wiley JL, Craft RM (2014) Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 143:22–28. https://doi.org/10.1016/j.drugalcdep.2014.07.029
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag. Vol, New York, p 2009
Wickham H, Averick M, Bryan J et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686
Wiley JL (2003) Sex-dependent effects of Δ9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett 352:77–80. https://doi.org/10.1016/j.neulet.2003.08.050
Williams NNB, Ewell TR, Abbotts KSS et al (2021) Comparison of five oral cannabidiol preparations in adult humans: pharmacokinetics, body composition, and heart rate variability. Pharm 14:35
Funding
This publication was supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (Colorado Clinical and Translational Sciences Institute Co-Pilot Grant Funds awarded to HK). HK is also supported by K23AA028238. MD is supported by F31AA030492. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karoly, H.C., Drennan, M.L., Prince, M.A. et al. Consuming oral cannabidiol prior to a standard alcohol dose has minimal effect on breath alcohol level and subjective effects of alcohol. Psychopharmacology 240, 1119–1129 (2023). https://doi.org/10.1007/s00213-023-06349-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06349-z