Abstract
Summary
Previously observational studies did not draw a clear conclusion on the association between fatty liver diseases and bone mineral density (BMD).
Our large-scale studies revealed that MAFLD and hepatic steatosis had no causal effect on BMD, while some metabolic factors were correlated with BMD.
The findings have important implications for the relationship between fatty liver diseases and BMD, and may help direct the clinical management of MAFLD patients who experience osteoporosis and osteopenia.
Purpose
Liver and bone are active endocrine organs with several metabolic functions. However, the link between metabolic dysfunction-associated fatty liver disease (MAFLD) and bone mineral density (BMD) is contradictory.
Methods
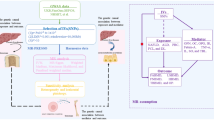
Using the UK Biobank and National Health and Nutrition Examination Survey (NHANES) dataset, we investigated the association between MAFLD, steatosis, and BMD in the observational analysis. We performed genome-wide association analysis to identify single-nucleotide polymorphisms associated with MAFLD. Large-scale two-sample Mendelian randomization (TSMR) analyses examined the potential causal relationship between MAFLD, hepatic steatosis, or major comorbid metabolic factors, and BMD.
Results
After adjusting for demographic factors and body mass index, logistic regression analysis demonstrated a significant association between MAFLD and reduced heel BMD. However, this association disappeared after adjusting for additional metabolic factors. MAFLD was not associated with total body, femur neck, and lumbar BMD in the NHANES dataset. Magnetic resonance imaging-measured steatosis did not show significant associations with reduced total body, femur neck, and lumbar BMD in multivariate analysis. TSMR analyses indicated that MAFLD and hepatic steatosis were not associated with BMD. Among all MAFLD-related comorbid factors, overweight and type 2 diabetes showed a causal relationship with increased BMD, while waist circumference and hyperlipidemia had the opposite effect.
Conclusion
No causal effect of MAFLD and hepatic steatosis on BMD was observed in this study, while some metabolic factors were correlated with BMD. This has important implications for understanding the relationship between fatty liver disease and BMD, which may help direct the clinical management of MAFLD patients with osteoporosis.
Graphical Abstract




Similar content being viewed by others
Data Availability
The UK Biobank data in the observational analysis is available on application (www.ukbiobank.co.uk). This research was conducted under application number 92668. The NHANES data can be found here: www.cdc.gov/nchs/nhanes/. The GWAS summary data are publicly available.
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- T2D:
-
Type 2 diabetes
- MAFLD:
-
Metabolic-associated fatty liver disease
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- MR:
-
Mendelian randomization
- TSMR:
-
Two-sample Mendelian randomization
- GWAS:
-
Genome-wide association study
- NHANES:
-
National Health and Nutrition Examination Survey
- UKBB:
-
UK Biobank
- FLI:
-
Fatty liver index
- MRI:
-
Magnetic resonance imaging
- MRI-PDFF:
-
Proton density fat fraction
- WC:
-
Waist circumference
- HDL-C:
-
Plasma high–density lipoprotein cholesterol
- DXA:
-
Dual-energy X-ray absorptiometry
- SD:
-
Standard deviation
- SNP:
-
Single nucleotide polymorphism
- IV:
-
Instrumental variable
- IVW:
-
Inverse variance weighted
- HBA1c:
-
Glycated hemoglobin
- LDL-C:
-
Plasma low–density lipoprotein cholesterol
- CHO:
-
Plasma total cholesterol
- TG:
-
Plasma triglycerides
- GGT:
-
Triglycerides and gamma-glutamyl transferase
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- LogOR:
-
Log-transformed OR
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
References
Younossi Z, Anstee QM, Marietti M et al (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15(1):11–20
Muzurović E, Mikhailidis DP, Mantzoros C (2021) Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism 119:154770
Yki-Järvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2(11):901–910
Eslam M, Sanyal AJ, George J (2020) MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158(7):1999-2014.e1991
Eslam M, Newsome PN, Sarin SK et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73(1):202–209
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359(9319):1761–1767
Chen DZ, Xu QM, Wu XX et al (2018) The combined effect of nonalcoholic fatty liver disease and metabolic syndrome on osteoporosis in postmenopausal females in Eastern China. Int J Endocrinol 2018:2314769
Lin S, Huang J, Wang M et al (2020) Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 40(9):2082–2089
Haukeland JW, Damås JK, Konopski Z et al (2006) Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 44(6):1167–1174
Xie R, Liu M (2022) Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol (Lausanne) 13:857110
Pan B, Cai J, Zhao P et al (2022) Relationship between prevalence and risk of osteoporosis or osteoporotic fracture with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Osteoporos Int 33(11):2275–2286
Shen Z, Cen L, Chen X et al (2020) Increased risk of low bone mineral density in patients with non-alcoholic fatty liver disease: a cohort study. Eur J Endocrinol 182(2):157–164
Mantovani A, Gatti D, Zoppini G et al (2019) Association between nonalcoholic fatty liver disease and reduced bone mineral density in children: a meta-analysis. Hepatology 70(3):812–823
Ciardullo S, Muraca E, Zerbini F et al (2021) NAFLD and liver fibrosis are not associated with reduced femoral bone mineral density in the general US population. J Clin Endocrinol Metab 106(8):e2856–e2865
Mantovani A, Dauriz M, Gatti D et al (2019) Systematic review with meta-analysis: non-alcoholic fatty liver disease is associated with a history of osteoporotic fractures but not with low bone mineral density. Aliment Pharmacol Ther 49(4):375–388
Xie R, Zhang Y, Yan T et al (2022) Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents. Medicine (Baltimore) 101(41):e31164
Bowden J, Holmes MV (2019) Meta-analysis and Mendelian randomization: a review. Res Synth Methods 10(4):486–496
Bedogni G, Bellentani S, Miglioli L et al (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6:33
Caussy C, Reeder SB, Sirlin CB et al (2018) Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH Trials. Hepatology 68(2):763–772
Eddowes PJ, Sasso M, Allison M et al (2019) Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156(6):1717–1730
Jiang L, Zheng Z, Fang H et al (2021) A generalized linear mixed model association tool for biobank-scale data. Nat Genet 53(11):1616–1621
Speliotes EK, Yerges-Armstrong LM, Wu J et al (2011) Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 7(3):e1001324
Morris JA, Kemp JP, Youlten SE et al (2019) An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 51(2):258–266
Medina-Gomez C, Kemp JP, Trajanoska K et al (2018) Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am J Hum Genet 102(1):88–102
Mahajan A, Wessel J, Willems SM et al (2018) Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet 50(4):559–571
Berndt SI, Gustafsson S, Mägi R et al (2013) Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 45(5):501–512
Shungin D, Winkler TW, Croteau-Chonka DC et al (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518(7538):187–196
Willer CJ, Schmidt EM, Sengupta S et al (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45(11):1274–1283
Manning AK, Hivert MF, Scott RA et al (2012) A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44(6):659–669
Evangelou E, Warren HR, Mosen-Ansorena D et al (2018) Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 50(10):1412–1425
Papadimitriou N, Dimou N, Tsilidis KK et al (2020) Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun 11(1):597
Lee SJ, Lee JY, Sung J (2019) Obesity and bone health revisited: a Mendelian randomization study for Koreans. J Bone Miner Res 34(6):1058–1067
Chen HJ, Yang HY, Hsueh KC et al (2018) Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: a population-based retrospective cohort study. Medicine (Baltimore) 97(42):e12835
Ahn SH, Seo DH, Kim SH et al (2018) The relationship between fatty liver index and bone mineral density in Koreans: KNHANES 2010–2011. Osteoporos Int 29(1):181–190
Wang Y, Wen G, Zhou R et al (2018) Association of nonalcoholic fatty liver disease with osteoporotic fractures: a cross-sectional retrospective study of Chinese individuals. Front Endocrinol (Lausanne) 9:408
Upala S, Jaruvongvanich V, Wijarnpreecha K et al (2017) Nonalcoholic fatty liver disease and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab 35(6):685–693
Lee DY, Park JK, Hur KY et al (2018) Association between nonalcoholic fatty liver disease and bone mineral density in postmenopausal women. Climacteric 21(5):498–501
Polyzos SA, Anastasilakis AD, Kountouras J et al (2016) Circulating sclerostin and Dickkopf-1 levels in patients with nonalcoholic fatty liver disease. J Bone Miner Metab 34(4):447–456
Wang N, Chen C, Zhao L et al (2018) Vitamin D and nonalcoholic fatty liver disease: bi-directional mendelian randomization analysis. EBioMedicine 28:187–193
Yuan S, Larsson SC (2023) Inverse association between serum 25-hydroxyvitamin D and nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 21(2):398–405.e394
Zhou H, Li C, Song W et al (2021) Increasing fasting glucose and fasting insulin associated with elevated bone mineral density-evidence from cross-sectional and MR studies. Osteoporos Int 32(6):1153–1164
Walsh JS, Vilaca T (2017) Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int 100(5):528–535
Guañabens N, Parés A (2018) Osteoporosis in chronic liver disease. Liver Int 38(5):776–785
Wang Y, Dai J, Zhong W et al (2018) Association between serum cholesterol level and osteoporotic fractures. Front Endocrinol (Lausanne) 9:30
Castera L, Friedrich-Rust M, Loomba R (2019) Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156(5):1264-1281.e1264
Acknowledgements
This research has been conducted using the UK Biobank (under application number 92668) and NHANES resource. We thank all the participants for their selfless contributions to the study. We thank all the genetics consortiums for making the GWAS summary data publicly available.
Funding
This work was supported by National Key Research and Development Program of China (No.2021YFC2500805, 2020YFC2006400), the National Nature Science Foundation of China (No.81972897,82172751), Guangzhou Science and Technology Project (No.202201011183), and GuangDong Basic and Applied Basic Research Foundation (No.2022A1515110656). This research has been conducted using the UK Biobank (under application number 92668) and NHANES resource. We thank all the participants for their selfless contributions to the study. We thank all the genetics consortiums for making the GWAS summary data publicly available.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards. Ethical approval was granted for the UK Biobank by the North West-Haydock Research Ethics Committee (REC reference: 16/NW/0274). This study was conducted using the UK Biobank resource under application number 92668.
Patient consent
All participants provided informed consent at baseline assessment in the UK Biobank and NHANES datasets.
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, L., Li, Y., Hong, C. et al. Association between fatty liver index and controlled attenuation parameters as markers of metabolic dysfunction-associated fatty liver disease and bone mineral density: observational and two-sample Mendelian randomization studies. Osteoporos Int 35, 679–689 (2024). https://doi.org/10.1007/s00198-023-06996-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06996-0