Abstract
Summary
Longitudinal studies of bone using high-resolution medical imaging may result in non-physiological measurements of longitudinal changes. In this study, we determined that three-dimensional image processing techniques best capture realistic longitudinal changes in bone density and should therefore be used with high-resolution imaging when studying bone changes over time.
Introduction
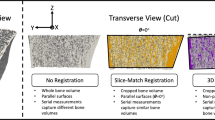
The purpose of this study was to determine which longitudinal analysis technique (no registration (NR), slice-match (SM) registration, or three-dimensional registration (3DR)) produced the most realistic longitudinal changes in a 3-year study of bone density and structure using high-resolution peripheral quantitative computed tomography (HR-pQCT).
Methods
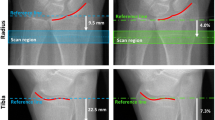
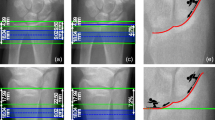
We assessed HR-pQCT scans of the distal radius and tibia for men and women (N = 40) aged 55–70 years at baseline and 6, 12, 24, and 36 months. To evaluate which longitudinal analysis technique (NR, SM, or 3DR) best captured physiologically reasonable 3-year changes, we calculated the standard deviation of the absolute rate of change in each bone parameter. The data were compared between longitudinal analysis techniques using repeated measures ANOVA and post hoc analysis.
Results
As expected, both SM and 3DR better captured physiological longitudinal changes than NR. At the tibia, there were no differences between SM and 3DR; however, at the radius where precision was lower, 3DR produced better results for total bone mineral density.
Conclusions
At least SM or 3DR should be implemented in longitudinal studies using HR-pQCT. 3DR is preferable, particularly at the radius, to ensure that physiological changes in bone density are observed.




Similar content being viewed by others
Data availability
The data are not publicly available. Upon reasonable requests to the corresponding author, data may be shared.
References
Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK (2011) Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res 26:50–62
Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJI (2006) Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21:124–131
Dalzell N, Kaptoge S, Morris N, Berthier A, Koller B, Braak L, van Rietbergen B, Reeve J (2009) Bone micro-architecture and determinants of strength in the radius and tibia: age-related changes in a population-based study of normal adults measured with high-resolution pQCT. Osteoporos Int 20:1683–1694
Burt L, Liang Z, Sajobi TT, Hanley DA, Boyd SK (2016) Sex- and site-specific normative data curves for HR-pQCT. J Bone Miner Res 31:2041–2047
Varga P, Baumbach S, Pahr D, Zysset PK (2009) Validation of an anatomy specific finite element model of Colles’ fracture. J Biomech 42:1726–1731
Christen D, Melton LJI, Zwahlen A, Shreyasee A, Khosla S, Müller R (2013) Improved fracture risk assessment based on nonlinear micro-finite element simulations from HRpQCT images at the distal radius. J Bone Miner Res 28:2601–2608
Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E, Berger C, Burt L, Chapurlat R, Chevalley T, Ferrari S, Goltzman D, Hanley DA, Hannan MT, Khosla S, Liu CT, Lorentzon M, Mellstrom D, Merle B, Nethander M, Rizzoli R, Sornay-Rendu E, van Rietbergen B, Sundh D, Wong AKO, Ohlsson C, Demissie S, Kiel DP, Bouxsein ML (2019) Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7:34–43
Rizzoli R, Chapurlat RD, Laroche J-M, Krieg MA, Thomas T, Frieling I, Boutroy S, Laib A, Bock O, Felsenberg D (2012) Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Osteoporos Int 23:305–315
Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25:1886–1894
Tsai JN, Nishiyama KK, Lin D, Yuan A, Lee H, Bouxsein ML, Leder BZ (2017) Effects of denosumab and teriparatide transitions on the bone microarchitecture and estimated strength: the DATA-Switch HR-pQCT study. J Bone Miner Res 32:2001–2009
Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK (2019) Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength. JAMA 322:736–745
Ellouz R, Chapurlat R, van Rietbergen B, Christen P, Pialat JB, Boutroy S (2014) Challenges in longitudinal measurements with HR-pQCT: evaluation of a 3D registration method to improve bone microarchitecture and strength measurement reproducibility. Bone 63:147–157
Boyd SK, Moser S, Kuhn M, Klinck RJ, Krauze PL, Müller R, Gasser JA (2006) Evaluation of three-dimensional image registration methodologies for in vivo micro-computed tomography. Ann Biomed Eng 34:1587–1599
Bonaretti S, Vilayphiou N, Chan CM et al (2017) Operator variability in scan positioning is a major component of HR-pQCT precision error and is reduced by standardized training. Osteoporos Int 28:247–257
Chiba K, Okazaki N, Kurogi A, Isobe Y, Yonekura A, Tomita M, Osaki M (2018) Precision of second-generation high-resolution peripheral quantitative computed tomography: intra- and intertester reproducibilities and factors involved in the reproducibility of cortical porosity. J Clin Densitom 21:295–302
MacNeil JA, Boyd SK (2008) Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 30:792–799
Shi L, Wang D, Hung VWY, Yeugn BHY, Griffith JF, Chu WCW, Heng PAH, Cheng JCY, Qin L (2010) Fast and accurate 3-D registration of HR-pQCT images. IEEE Trans Inf Technol Biomed 14:1291–1297
Waarsing JH, Day JS, van der Linden JC, Ederveen AG, Spanjers C, De Clerck N, Sasov A, Verhaar JAN, Weinans H (2004) Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone 34:163–169
Nishiyama KK, Campbell GM, Klinck RJ, Boyd SK (2010) Reproducibility of bone micro-architecture measurements in rodents by in vivo micro-computed tomography is maximized with three-dimensional image registration. Bone 46:155–161
Lan S, Luo S, Huh BK, Chandra A, Altman AR, Qin L, Liu XS (2013) 3D image registration is critical to ensure accurate detection of longitudinal changes in trabecular bone density, microstructure, and stiffness measurements in rat tibiae by in vivo microcomputed tomography (muCT). Bone 56:83–90
Campbell GM, Tiwari S, Grundmann F, Purcz N, Schem C, Glüer C-C (2014) Three-dimensional image registration improves the long-term precision of in vivo micro-computed tomographic measurements in anabolic and catabolic mouse models. Calcif Tissue Int 94:282–292
Goshtasby A (2005) 2-D And 3-D Image Registration For Medical, Remote Sensing, And Industrial Applications. Hoboken, N.J.: Wiley. pp 63–154
Burt LA, Gaudet S, Kan M, Rose MS, Billington EO, Boyd SK, Hanley DA (2018) Methods and procedures for: a randomized double-blind study investigating dose-dependent longitudinal effects of vitamin D supplementation on bone health. Contemp Clin Trials 67:68–73
Pauchard Y, Liphardt AM, Macdonald HM, Hanley DA, Boyd SK (2012) Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone 50:1304–1310
Manske SL, Davison EM, Burt LA, Raymond DA, Boyd SK (2017) The estimation of second-generation HR-pQCT from first-generation HR-pQCT using in vivo cross-calibration. J Bone Miner Res 32:1514–1524
Manske SL, Zhu Y, Sandino C, Boyd SK (2015) Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone 79:213–221
Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S (2010) Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res 25:983–993
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41:505–515
Glüer C-C (1999) Monitoring skeletal changes by radiological techniques. J Bone Miner Res 14:1952–1962
Bonnick SL, Johnston CC Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E (2001) Importance of precision in bone density measurements. J Clin Densitom 4:105–110
Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK (2010) Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 47:519–528
Burghardt AJ, Pialat J-B, Kazakia GJ, Boutroy S, Engelke K, Patsch JM, Valentinitsch A, Liu D, Szabo E, Bogado CE, Zanchetta MB, McKay HA, Shane E, Boyd SK, Bouxsein ML, Chapurlat R, Khosla S, Majumdar S (2013) Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J Bone Miner Res 28:524–536
Boyd SK (2008) Site-specific variation of bone micro-architecture in the distal radius and tibia. J Clin Densitom 11:424–430
Pauchard Y, Ayres FJ, Boyd SK (2011) Automated quantification of three-dimensional subject motion to monitor image quality in high-resolution peripheral quantitative computed tomography. Phys Med Biol 56:6523–6543
Acknowledgments
The authors thank J.M. Allan, J.A. Allan, S. Gaudet, M. Kan, and B. Love for participant recruitment and A. Cooke, S. Kwong, and D. Raymond for scan acquisition and analysis. The authors also thank the study participants for generously donating their time to support our research.
Funding
This study was funded by Pure North S’Energy Foundation in response to an investigator-initiated research proposal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Conjoint Health Research Ethics Board at the University of Calgary and Health Canada. All participants gave written consent before participating in the study.
Conflicts of interest
TDK, CMJdB, LG, DAH, and LAB have nothing to declare. EOB has previously received honoraria from Amgen and Eli Lilly and a research grant from Amgen. SKB has received honorariums from Amgen and Servier and is co-owner of Numerics88 Solutions Inc.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kemp, T., de Bakker, C., Gabel, L. et al. Longitudinal bone microarchitectural changes are best detected using image registration. Osteoporos Int 31, 1995–2005 (2020). https://doi.org/10.1007/s00198-020-05449-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05449-2