Abstract
Aims
Lactate accumulation is reported to be a biomarker for diabetic nephropathy progression. Lactate drives lysine lactylation, a newly discovered post-translational modification that is involved in the pathogenesis of cancers and metabolic and inflammatory disease. Here, we aimed to determine whether lysine lactylation is involved in the pathogenesis of diabetic nephropathy.
Methods
Renal biopsy samples from individuals with diabetic nephropathy (n=22) and control samples from individuals without diabetes and kidney disease (n=9) were obtained from the First Affiliated Hospital of Zhengzhou University for immunohistochemical staining. In addition, we carried out global lactylome profiling of kidney tissues from db/m and db/db mice using LC-MS/MS. Furthermore, we assessed the role of lysine lactylation and acyl-CoA synthetase family member 2 (ACSF2) in mitochondrial function in human proximal tubular epithelial cells (HK-2).
Results
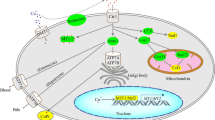
The expression level of lysine lactylation was significantly increased in the kidneys of individuals with diabetes as well as in kidneys from db/db mice. Integrative lactylome analysis of the kidneys of db/db and db/m mice identified 165 upregulated proteins and 17 downregulated proteins, with an increase in 356 lysine lactylation sites and a decrease in 22 lysine lactylation sites decreased. Subcellular localisation analysis revealed that most lactylated proteins were found in the mitochondria (115 proteins, 269 sites). We further found that lactylation of the K182 site in ACSF2 contributes to mitochondrial dysfunction. Finally, the expression of ACSF2 was notably increased in the kidneys of db/db mice and individuals with diabetic nephropathy.
Conclusions
Our study strongly suggests that lysine lactylation and ACSF2 are mediators of mitochondrial dysfunction and may contribute to the progression of diabetic nephropathy.
Data availability
The LC-MS/MS proteomics data have been deposited in the ProteomeXchange Consortium database (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD050070.
Graphical Abstract









Similar content being viewed by others
Abbreviations
- ACSF2:
-
Acyl-CoA synthetase family member 2
- DRP1:
-
Dynamin-related protein 1
- ESRD:
-
End-stage renal disease
- FerrDb:
-
Ferroptosis database
- HBSS:
-
Hanks’ balanced salt solution
- IHC:
-
Immunohistochemical
- K182la:
-
Lysine lactylation at site 182
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- Kla:
-
Lysine lactylation
- LCFA:
-
Long-chain fatty acids
- MFN2:
-
Mitofusin 2
- Pan-Kla:
-
Pan-antibody of lactylation
- PAS:
-
Periodic acid–Schiff
- PI3K:
-
Phosphoinositide 3-kinase
- PMSF:
-
Phenylmethylsulfonyl fluoride
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid
- TFAM:
-
Mitochondrial transcription factor A
- UACR:
-
Urine albumin/creatinine ratio
- WT:
-
Wild-type
References
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
Cheng HT, Xu X, Lim PS, Hung KY (2021) Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care 44(1):89–97. https://doi.org/10.2337/dc20-1913
Fioretto P, Pontremoli R (2022) Expanding the therapy options for diabetic kidney disease. Nat Rev Nephrol 18(2):78–79. https://doi.org/10.1038/s41581-021-00522-3
Lee DY, Kim JY, Ahn E et al (2022) Associations between local acidosis induced by renal LDHA and renal fibrosis and mitochondrial abnormalities in patients with diabetic kidney disease. Transl Res 249:88–109. https://doi.org/10.1016/j.trsl.2022.06.015
Kugathasan L, Darshi M, Srinivasan Sridhar V et al (2023) 12-OR: Urine lactate is a disease biomarker for diabetic kidney disease. Diabetes 72(Supplement_1):12-OR. https://doi.org/10.2337/db23-12-OR
Azushima K, Kovalik JP, Yamaji T et al (2023) Abnormal lactate metabolism is linked to albuminuria and kidney injury in diabetic nephropathy. Kidney Int 104(6):1135–1149. https://doi.org/10.1016/j.kint.2023.08.006
Wang N, Wang W, Wang X et al (2022) Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res 131(11):893–908. https://doi.org/10.1161/circresaha.122.320488
Xiong J, He J, Zhu J et al (2022) Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell 82(9):1660-1677.e10. https://doi.org/10.1016/j.molcel.2022.02.033
Yu J, Chai P, Xie M et al (2021) Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol 22(1):85. https://doi.org/10.1186/s13059-021-02308-z
Zhang D, Tang Z, Huang H et al (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574(7779):575–580. https://doi.org/10.1038/s41586-019-1678-1
Xie B, Lin J, Chen X et al (2023) CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol Cancer 22(1):151. https://doi.org/10.1186/s12943-023-01856-1
Jia M, Yue X, Sun W et al (2023) ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci Adv 9(22):eadg4993. https://doi.org/10.1126/sciadv.adg4993
Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C (2020) TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci U S A 117(48):30628–30638. https://doi.org/10.1073/pnas.2009778117
Wang J, Yang P, Yu T et al (2022) Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci 18(16):6210–6225. https://doi.org/10.7150/ijbs.75434
Yang Z, Yan C, Ma J et al (2023) Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab 5(1):61–79. https://doi.org/10.1038/s42255-022-00710-w
Yang D, Yin J, Shan L, Yi X, Zhang W, Ding Y (2022) Identification of lysine-lactylated substrates in gastric cancer cells. iScience 25(7):104630. https://doi.org/10.1016/j.isci.2022.104630
Wang X, Fan W, Li N et al (2023) YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol 24(1):87. https://doi.org/10.1186/s13059-023-02931-y
An S, Yao Y, Hu H et al (2023) PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis 14(7):457. https://doi.org/10.1038/s41419-023-05952-4
Xue M, Fang T, Sun H et al (2021) PACS-2 attenuates diabetic kidney disease via the enhancement of mitochondria-associated endoplasmic reticulum membrane formation. Cell Death Dis 12(12):1107. https://doi.org/10.1038/s41419-021-04408-x
Gan B (2021) Mitochondrial regulation of ferroptosis. J Cell Biol 220(9):e202105043. https://doi.org/10.1083/jcb.202105043
Gao M, Yi J, Zhu J et al (2019) Role of mitochondria in ferroptosis. Mol Cell 73(2):354-363.e353. https://doi.org/10.1016/j.molcel.2018.10.042
Naaman SC, Bakris GL (2023) Diabetic nephropathy: update on pillars of therapy slowing progression. Diabetes Care 46(9):1574–1586. https://doi.org/10.2337/dci23-0030
Forbes JM, Thorburn DR (2018) Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 14(5):291–312. https://doi.org/10.1038/nrneph.2018.9
Han Y, Xu X, Tang C et al (2018) Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol 16:32–46. https://doi.org/10.1016/j.redox.2018.02.013
Li Q, Liao J, Chen W et al (2022) NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic Biol Med 187:158–170. https://doi.org/10.1016/j.freeradbiomed.2022.05.024
Jiang N, Zhao H, Han Y et al (2020) HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif 53(11):e12909. https://doi.org/10.1111/cpr.12909
Liu L, Bai F, Song H et al (2022) Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol 50:102260. https://doi.org/10.1016/j.redox.2022.102260
Yang K, Fan M, Wang X et al (2022) Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ 29(1):133–146. https://doi.org/10.1038/s41418-021-00841-9
Stockwell BR (2022) Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185(14):2401–2421. https://doi.org/10.1016/j.cell.2022.06.003
Fang X, Ardehali H, Min J, Wang F (2022) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20(1):7–23. https://doi.org/10.1038/s41569-022-00735-4
Wang H, Cheng Y, Mao C et al (2021) Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther 29(7):2185–2208. https://doi.org/10.1016/j.ymthe.2021.03.022
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282. https://doi.org/10.1038/s41580-020-00324-8
Guo R, Duan J, Pan S et al (2023) The road from AKI to CKD: molecular mechanisms and therapeutic targets of ferroptosis. Cell Death Dis 14(7):426. https://doi.org/10.1038/s41419-023-05969-9
Zhao Z, Wu J, Xu H et al (2020) XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis 11(8):629. https://doi.org/10.1038/s41419-020-02871-6
Tonnus W, Meyer C, Steinebach C et al (2021) Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat Commun 12(1):4402. https://doi.org/10.1038/s41467-021-24712-6
Wang J, Liu Y, Wang Y, Sun L (2021) The cross-link between ferroptosis and kidney diseases. Oxid Med Cell Longev 2021:6654887. https://doi.org/10.1155/2021/6654887
Li S, Zheng L, Zhang J, Liu X, Wu Z (2021) Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med 162:435–449. https://doi.org/10.1016/j.freeradbiomed.2020.10.323
Kim S, Kang SW, Joo J et al (2021) Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis 12(2):160. https://doi.org/10.1038/s41419-021-03452-x
Watkins PA, Maiguel D, Jia Z, Pevsner J (2007) Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res 48(12):2736–2750. https://doi.org/10.1194/jlr.M700378-JLR200
Luo L, Zhang S, Guo N, Li H, He S (2023) ACSF2-mediated ferroptosis is involved in ulcerative colitis. Life Sci 313:121272. https://doi.org/10.1016/j.lfs.2022.121272
Wu MN, Zhou DM, Jiang CY et al (2022) Genetic analysis of potential biomarkers and therapeutic targets in ferroptosis from psoriasis. Front Immunol 13:1104462. https://doi.org/10.3389/fimmu.2022.1104462
Shi H, Qi H, Xie D et al (2023) Inhibition of ACSF2 protects against renal ischemia/reperfusion injury via mediating mitophagy in proximal tubular cells. Free Radic Biol Med 198:68–82. https://doi.org/10.1016/j.freeradbiomed.2023.02.003
Wang M, Su Y, Hou C et al (2022) Targeted lipidomics analysis of lysine 179 acetylation of ACSF2 in rat hepatic stellate cells. Prostaglandins Other Lipid Mediat 163:106671. https://doi.org/10.1016/j.prostaglandins.2022.106671
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Acknowledgements
We thank the Biobank of the First Affiliated Hospital of Zhengzhou University (China Human Genetic Resources Preservation Approval No. [2022]BC0079) for providing human control kidney tissues.
Data availability
The LC-MS/MS proteomics data have been deposited in the ProteomeXchange Consortium database (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD050070.
Funding
This study is supported by grants from the National Natural Science Foundation of China General Project (no. 81970633), National Natural Science Foundation of China Young Scientists Project (no. 82200796), Medical Science and Technology Research Project of Henan Province (SBGJ202102145) and Natural Science Foundation of Henan Province Excellent Young Scientists Fund Program (no. 202300410363).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
DL, ZsL and ZhL designed the study; JC, QF, YQ and SP conducted the experiments. LL, YL and XZ were responsible for feeding the mice, measuring weight and blood glucose levels and collecting kidney tissues and serum samples. LL, YL and XZ collected samples of human kidney tissues. YQ, LL,YL and XZ conducted the statistical analysis. JC and QF wrote the manuscript and SP, LL and YL drew the figures. YQ, SP, LL, YL and XZ contributed to drafting the manuscript. JC, QF, LL, DL, ZhL and ZsL revised the manuscript. All authors approved the final version of the manuscript. ZsL is the guarantor of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Feng, Q., Qiao, Y. et al. ACSF2 and lysine lactylation contribute to renal tubule injury in diabetes. Diabetologia (2024). https://doi.org/10.1007/s00125-024-06156-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00125-024-06156-x