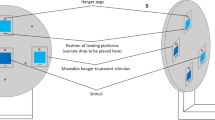
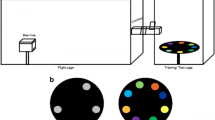
Abstract
Despite representing the majority of bee species, non-eusocial bees (e.g. solitary, subsocial, semisocial, and quasisocial species) are comparatively understudied in learning, memory, and cognitive-like behaviour compared to eusocial bees, such as honeybees and bumblebees. Ecologically relevant colour discrimination tasks are well-studied in eusocial bees, and research has shown that a few non-eusocial bee species are also capable of colour learning and long-term memory retention. Australia hosts over 2000 native bee species, most of which are non-eusocial, yet evidence of cognitive-like behaviour and learning abilities under controlled testing conditions is lacking. In the current study, I examine the learning ability of a non-eusocial Australian bee, Lasioglossum (Chilalictus) lanarium, using aversive differential conditioning during a colour discrimination task. L. lanarium learnt to discriminate between salient blue- and yellow-coloured stimuli following training with simulated predation events. This study acts as a bridge between cognitive studies on eusocial and non-social bees and introduces a framework for testing non-eusocial wild bees on elemental visual learning tasks using aversive conditioning. Non-eusocial bee species are far more numerous than eusocial species and contribute to agriculture, economics, and ecosystem services in Australia and across the globe. Thus, it is important to study their capacity to learn flower traits allowing for successful foraging and pollination events, thereby permitting us a better understanding of their role in plant-pollinator interactions.


Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Code availability
Available upon request.
References
Amaya-Marquez M, Wells H (2008) Social complexity and learning foraging tasks in bees. Caldasia 30:469–477 Atlas of Living Australia website. Species page: https://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:8634ccfb-2dea-48d4-8853-6f91018440f2. Accessed 16th February 2021
Avarguès-Weber A, de Brito Sanchez MG, Giurfa M, Dyer AG (2010a) Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS ONE 5:e15370–e15370
Avarguès-Weber A, Portelli G, Benard J, Dyer A, Giurfa M (2010b) Configural processing enables discrimination and categorization of face-like stimuli in honeybees. J Exp Biol 213:593–601
Avarguès-Weber A, Dyer AG, Giurfa M (2011) Conceptualization of above and below relationships by an insect. Proc Biol Sci 278:898–905
Avarguès-Weber A, Giurfa M (2013) Conceptual learning by miniature brains. Proc Biol Sci 280:20131907
Avarguès-Weber A, d’Amaro D, Metzler M, Dyer AG (2014) Conceptualization of relative size by honeybees. Front Behav Neurosci 8:1–8
Avarguès-Weber A, Dyer AG, Ferrah N, Giurfa M (2015) The forest or the trees: preference for global over local image processing is reversed by prior experience in honeybees. Proc Biol Sci 282:20142384
Avargues-Weber A, d’Amaro D, Metzler M, Finke V, Baracchi D, Dyer AG (2018) Does holistic processing require a large brain? Insights from honeybees and wasps in fine visual recognition tasks. Front Psychol 9:1313
Bar-Shai N, Keasar T, Shmida A (2011) How do solitary bees forage in patches with a fixed number of food items? Anim Behav 82:1367–1372
Barth FG (1985) Insects and flowers. The biology of a partnership. Princeton University Press USA, Princeton
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823:1-51
Batley M, Hogendoorn K (2009) Diversity and conservation status of native Australian bees. Apidologie 40:347–354
Bell M, Spooner-Hart R, Haigh AM (2006) Pollination of greenhouse tomatoes by the Australian bluebanded bee Amegilla (Zonamegilla) holmesi (Hymenoptera: Apidae). J Econ Entomol 99:437–442
Bortot M, Agrillo C, Avarguès-Weber A, Bisazza A, Miletto Petrazzini ME, Giurfa M (2019a) Honeybees use absolute rather than relative numerosity in number discrimination. Biol Let 15:20190138
Bortot M, Stancher G, Vallortigara G (2019b) Transfer from number to size reveals abstract coding of magnitude in honeybees. IScience 23:101122
Bray R (1973) Characteristics of some bees of the family Megachilidae in Southeast Queensland and their potential as lucerne pollinators. Aust J Entomol 12:99–102
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510
Brown MJ, Paxton RJ (2009) The conservation of bees: a global perspective. Apidologie 40:410–416
Buatois A, Flumian C, Schultheiss P, Avarguès-Weber A, Giurfa M (2018) Transfer of visual learning between a virtual and a real environment in honey bees: the role of active vision. Front Behav Neurosci 12:139
Chittka L (1992) The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A 170:533–543
Chittka L (2017) Bee cognition. Curr Biol 27:R1049–R1053
Chittka L, Geiger K (1995) Can honey bees count landmarks? Anim Behav 49:159–164
Chittka L, Dyer A (2012) Cognition: Your face looks familiar. Nature 481:154–155
Chittka L, Beier W, Hertel H, Steinmann E, Menzel R (1992) Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in Hymenoptera. J Comp Physiol A 170:545–563
Chittka L, Dyer AG, Bock F, Dornhaus A (2003) Psychophysics: bees trade off foraging speed for accuracy. Nature 424:388–388
Collett T, Fry S, Wehner R (1993) Sequence learning by honeybees. J Comp Physiol A 172:693–706
Dacke M, Srinivasan MV (2008) Evidence for counting in insects. Anim Cogn 11:683–689
Danforth BN, Ji S (2001) Australian Lasioglossum + Homalictus form a monophyletic group: resolving the “Australian enigma”. Syst Biol 50:268–283
Dukas R, Real LA (1991) Learning foraging tasks by bees: a comparison between social and solitary species. Anim Behav 42:269–276
Dyer AG (2012) The mysterious cognitive abilities of bees: why models of visual processing need to consider experience and individual differences in animal performance. J Exp Biol 215:387–395
Dyer AG, Chittka L (2004) Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. J Comp Physiol A 190:759–763
Dyer AG, Neumeyer C (2005) Simultaneous and successive colour discrimination in the honeybee (Apis mellifera). J Comp Physiol A 191:547–557
Dyer AG, Griffiths DW (2012) Seeing near and seeing far; behavioural evidence for dual mechanisms of pattern vision in the honeybee (Apis mellifera). J Exp Biol 215:397–404
Dyer AG, Arikawa K (2014) A hundred years of color studies in insects: with thanks to Karl von Frisch and the workers he inspired. J Comp Physiol A 200:409–410
Dyer AG, Neumeyer C, Chittka L (2005) Honeybee (Apis mellifera) vision can discriminate between and recognise images of human faces. J Exp Biol 208:4709–4714
Dyer AG, Spaethe J, Prack S (2008) Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J Comp Physiol A 194:617–627
Efler D, Ronacher B (2000) Evidence against a retinotopic-template matching in honeybees’ pattern recognition. Vision Res 40:3391–3403
Garcia JE, Shrestha M, Howard SR, Petersen P, Dyer AG (2018) Signal or cue: the role of structural colors in flower pollination. Curr Zool 65:467–481
Giurfa M, Hammer M, Stach S, Stollhoff N, Müller-Deisig N, Mizyrycki C (1999) Pattern learning by honeybees: conditioning procedure and recognition strategy. Anim Behav 57:315–324
Giurfa M, Nunez J, Chittka L, Menzel R (1995) Colour preferences of flower-naive honeybees. J Comp Physiol A 177:247–259
Giurfa M, Eichmann B, Menzel R (1996) Symmetry perception in an insect. Nature 382:458–461
Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV (2001) The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 410:930–933
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Heard TA (1999) The role of stingless bees in crop pollination. Annu Rev Entomol 44:183–206
Herrera CM (2020) Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years. Proc R Soc B 287:20192657
Hogendoorn K, Keller M (2012) Native Australian bees as potential pollinators of lucerne (Publication No 12/048; Project No. PRJ-005657). Rural Industries Research and Development Corporation. Canberra, Australia
Hogendoorn K, Steen Z, Schwarz MP (2000) Native Australian carpenter bees as a potential alternative to introducing bumble bees for tomato pollination in greenhouses. J Apic Res 39:67–74
Hogendoorn K, Gross CL, Sedgley M, Keller MA (2006) Increased tomato yield through pollination by native Australian Amegilla chlorocyanea (Hymenoptera: Anthophoridae). J Econ Entomol 99:828–833
Hogendoorn K, Bartholomaeus F, Keller MA (2010) Chemical and sensory comparison of tomatoes pollinated by bees and by a pollination wand. J Econ Entomol 103:1286–1292
Hogendoorn K, Coventry S, Keller MA (2007) Foraging behaviour of a blue banded bee, Amegilla chlorocyanea in greenhouses: implications for use as tomato pollinators. Apidologie 38:86–92
Horridge GA (1997) Pattern discrimination by the honeybee: disruption as a cue. J Comp Physiol A 181:267–277
Houston T (2018) A guide to native bees of Australia. CSIRO Publishing, Australia
Howard SR, Garcia JE, Dyer AG (2021) Comparative psychophysics of colour preferences in two species of non-eusocial Australian native halictid bees. J Comp Physiol A
Howard SR, Avarguès-Weber A, Garcia J, Dyer AG (2017a) Free-flying honeybees extrapolate relational size rules to sort successively visited artificial flowers in a realistic foraging situation. Anim Cogn 20:627–638
Howard SR, Avarguès-Weber A, Garcia JE, Stuart-Fox D, Dyer AG (2017b) Perception of contextual size illusions by honeybees in restricted and unrestricted viewing conditions. Proc Biological Sci 284:20172278
Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2018a) Numerical ordering of zero in honey bees. Science 360:1124–1126
Howard SR, Shrestha M, Schramme J, Garcia JE, Avarguès-Weber A, Greentree AD, Dyer AG (2018b) Honeybees prefer novel insect-pollinated flower shapes over bird-pollinated flower shapes. Curr Zool 65:457–465
Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2019a) Achieving arithmetic learning in honeybees and examining how individuals learn. Commun Integr Biol 12:166–170
Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2019b) Numerical cognition in honeybees enables addition and subtraction. Sci Adv 5:easv0961
Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2019c) Surpassing the subitizing threshold: appetitive–aversive conditioning improves discrimination of numerosities in honeybees. J Exp Biol 222:jeb205658
Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG (2019d) Symbolic representation of numerosity by honeybees (Apis mellifera): Matching characters to small quantities. Proc Biological Sci 286:20190238
Howard SR, Schramme J, Garcia JE, Ng L, Avarguès-Weber A, Greentree AD, Dyer AG (2020) Spontaneous quantity discrimination of artificial flowers by foraging honeybees. J Exp Biol 223:jeb223610
Ings T, Wang M-Y, Chittka L (2012) Colour-independent shape recognition of cryptic predators by bumblebees. Behav Ecol Sociobiol 66:487–496
Ings TC, Chittka L (2009) Predator crypsis enhances behaviourally mediated indirect effects on plants by altering bumblebee foraging preferences. Proc Biol Sci 276:2031–2036
Ings TC, Chittka L (2008) Speed-accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr Biol 18:1520–1524
Jones EI, Dornhaus A (2011) Predation risk makes bees reject rewarding flowers and reduce foraging activity. Behav Ecol Sociobiol 65:1505–1511
Kleijn D et al (2015) Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun 6:7414
Lehrer M, Horridge G, Zhang S, Gadagkar R (1995) Shape vision in bees: innate preference for flower-like patterns. Series B: Biological Sciences 347:123–137
Loukola OJ, Gatto E, Híjar-Islas AC, Chittka L (2020) Selective interspecific information use in the nest choice of solitary bees. Anim Biol 70:215–225
Mallinger RE, Gaines-Day HR, Gratton C (2017) Do managed bees have negative effects on wild bees?: a systematic review of the literature. PloS One 12:e0189268
Marchal P et al (2019) Inhibitory learning of phototaxis by honeybees in a passive-avoidance task. Learn Mem 26:412–423
Martin NH (2004) Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus (Scrophulariaceae). Evol Ecol Res 6:777–782
Menzel R, Steinmann E, De Souza J, Backhaus W (1988) Spectral sensitivity of photoreceptors and colour vision in the solitary bee, Osmia rufa. J Exp Biol 136:35–52
Mitchell RJ, Irwin RE, Flanagan RJ, Karron JD (2009) Ecology and evolution of plant–pollinator interactions. Ann Bot 103:1355–1363
Nouvian M, Galizia CG (2019) Aversive training of honey bees in an automated Y-maze. Front Physiol 10:678
Orth AI, Waddington KD (1997) Hierarchical use of information by nectar-foraging carpenter bees on vertical inflorescences: floral color and spatial position. Israel J Plant Sci 45:213–221
Owens A, Cochard P, Durrant J, Perkin E, Seymoure B (2020) Light pollution is a driver of insect declines. Biol Conserv 241:108259
Perez SM, Waddington KD (1996) Carpenter bee (Xylocopa micans) risk indifference and a review of nectarivore risk-sensitivity studies. Am Zool 36:435–446
Perry CJ, Barron AB (2013) Honey bees selectively avoid difficult choices. Proc Natl Acad Sci 110:19155–19159
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Raguso RA (2008) Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Syst 39:549–569
Sands DP (2018) Important issues facing insect conservation in Australia: now and into the future. Austral Entomol 57:150–172
Santos AA, Leijs R, Picanço MC, Glatz R, Hogendoorn K (2020) Modelling the climate suitability of green carpenter bee (Xylocopa aerata) and its nesting hosts under current and future scenarios to guide conservation efforts. Austral Ecol 45:271–282
Sargent RD, Ackerly DD (2008) Plant–pollinator interactions and the assembly of plant communities. Trends Ecol Evol 23:123–130
Somanathan H, Borges RM, Warrant EJ, Kelber A (2008) Nocturnal bees learn landmark colours in starlight. Curr Biol 18:R996–R997
Somanathan H, Saryan P, Balamurali G (2019) Foraging strategies and physiological adaptations in large carpenter bees. J Comp Physiol A 205:387–398
Srinivasan MV (2010) Honey bees as a model for vision, perception, and cognition. Annu Rev Entomol 55:267–284
Taylor GS, Braby MF, Moir ML, Harvey MS, Sands DP, New TR, Kitching RL, McQuillan PB, Hogendoorn K, Glatz RV (2018) Strategic national approach for improving the conservation management of insects and allied invertebrates in Australia. Austral Entomol 57:124–149
Vergoz V, Roussel E, Sandoz J-C, Giurfa M (2007) Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PloS One 2:e288
Wang M-Y, Ings TC, Proulx MJ, Chittka L (2013) Can bees simultaneously engage in adaptive foraging behaviour and attend to cryptic predators? Anim Behav 86:859–866
Zhang E, Nieh JC (2015) The neonicotinoid imidacloprid impairs honey bee aversive learning of simulated predation. J Exp Biol 218:3199–3205
Zhang S, Srinivasan M (1994) Prior experience enhances pattern discrimination in insect vision. Nature 368:330–332
Zhang S, Bartsch K, Srinivasan M (1996) Maze learning by honeybees. Neurobiol Learn Mem 66:267–282
Zhang SW, Lehrer M, Srinivasan MV (1999) Honeybee memory: navigation by associative grouping and recall of visual stimuli. Neurobiol Learn Mem 72:180–201
Zhang S, Mizutani A, Srinivasan MV (2000) Maze navigation by honeybees: learning path regularity. Learn Mem 7:363–374
Acknowledgements
SRH thanks Associate Professor Matthew Symonds for his comments on the study and the editors and two anonymous reviewers for their constructive and helpful suggestions. SRH acknowledges funding from Deakin University.
Funding
SRH acknowledges funding from the Alfred Deakin Postdoctoral Research Fellowship.
Author information
Authors and Affiliations
Contributions
SRH performed data collection, analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal care was in accordance with institutional guidelines. Formal ethics approval was not required for invertebrate behavioural testing.
Conflicts of interest
The author declares no competing interests.
Additional information
Communicated by: Sean O’Donnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Online Resource 1 (ESM 1): Video showing the pre-test of a L. lanarium bee. (MP4 9295 KB)
Rights and permissions
About this article
Cite this article
Howard, S.R. Wild non-eusocial bees learn a colour discrimination task in response to simulated predation events. Sci Nat 108, 28 (2021). https://doi.org/10.1007/s00114-021-01739-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-021-01739-9