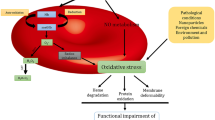
Abstract
The red blood cells (RBCs) are essential to transport oxygen (O2) and nutrients throughout the human body. Changes in the structure or functioning of the erythrocytes can lead to several deficiencies, such as hemolytic anemias, in which an increase in reactive oxidative species generation is involved in the pathophysiological process, playing a significant role in the severity of several clinical manifestations. There are important lines of defense against the damage caused by oxidizing molecules. Among the antioxidant molecules, the enzyme peroxiredoxin (Prx) has the higher decomposition power of hydrogen peroxide, especially in RBCs, standing out because of its abundance. This review aimed to present the recent findings that broke some paradigms regarding the three isoforms of Prxs found in RBC (Prx1, Prx2, and Prx6), showing that in addition to their antioxidant activity, these enzymes may have supplementary roles in transducing peroxide signals, as molecular chaperones, protecting from membrane damage, and maintenance of iron homeostasis, thus contributing to the overall survival of human RBCs, roles that seen to be disrupted in hemolytic anemia conditions.






Similar content being viewed by others
Availability of data and material
Not applicable.
References
Sankaran VG, Weiss MJ (2015) Anemia: progress in molecular mechanisms and therapies. Nat Med 21:221–230. https://doi.org/10.1038/nm.3814.Anemia
Nandakumar SK, Ulirsch JC, Sankaran VG (2016) Advances in understanding erythropoiesis: evolving perspectives. Br J Haematol 173:206–218. https://doi.org/10.1111/bjh.13938
Grace RF, Glader B (2018) Red blood cell enzyme disorders. Pediatr Clin North Am 65:579–595. https://doi.org/10.1016/j.pcl.2018.02.005
Mohandas N (2018) Inherited hemolytic anemia: a possessive beginner’s guide. Hematol Am Soc Hematol Educ Progr 2018:377–381. https://doi.org/10.1182/asheducation-2018.1.377
Andolfo I, Russo R, Gambale A, Iolascon A (2016) New insights on hereditary erythrocyte membrane defects. Haematologica 101:1284–1294. https://doi.org/10.3324/haematol.2016.142463
Wahed A, Quesada A, Dasgupta A (2020) Hemoglobinopathies and thalassemias. In: Hematology and Coagulation (Second Edition), 2nd ed. Academic Press. 51–75
Luzzatto L (2021) Diagnosis and clinical management of enzymopathies. Hematol Am Soc Hematol Educ Progr 2021:341–352. https://doi.org/10.1182/hematology.2021000266
Risinger M, Emberesh M, Kalfa TA (2019) Rare hereditary hemolytic anemias management: diagnostic approach and considerations in management. Hematol Oncol Clin North Am 33:373–392. https://doi.org/10.1016/j.hoc.2019.01.002
van Zwieten R, Verhoeven AJ, Roos D (2013) Inborn defects in the anti-oxidant systems of human red blood cells. Free Radic Biol Med 67:377–386. https://doi.org/10.1016/j.freeradbiomed.2013.11.022
Rocha S, Rocha-Pereira P, Cleto E et al (2020) Linkage of typically cytosolic peroxidases to erythrocyte membrane – a possible mechanism of protection in Hereditary Spherocytosis. BBA - Biomembr 1862:183172. https://doi.org/10.1016/j.bbamem.2019.183172
Flatt JF, Stevens-hernandez CJ, Cogan NM et al (2020) Expression of South East Asian ovalocytic band 3 disrupts erythroblast cytokinesis and reticulocyte maturation. Front Physiol 11:357. https://doi.org/10.3389/fphys.2020.00357
Gurbuz N, Yalcin O, Aksu TA, Baskurt OK (2004) The relationship between the enzyme activity, lipid peroxidation and red blood cells deformability in hemizygous and heterozygous glucose-6-phosphate dehydrogenase deficient individuals. Clin Hemorheol Microcirc 31:235–242
Fattizzo B, Cavallaro F, Marcello APML et al (2022) Pyruvate kinase deficiency: current challenges and future prospects. J Blood Med 13:461–471. https://doi.org/10.2147/JBM.S353907
Harteveld CL, Achour A, Arkesteijn SJG et al (2022) The hemoglobinopathies, molecular disease mechanisms and diagnostics. Int J Lab Hematol 44:28–36. https://doi.org/10.1111/ijlh.13885
Chaichompoo P, Svasti S, Smith DR (2022) The roles of mitophagy and autophagy in ineffective erythropoiesis in β-thalassemia. Int J Mol Sci 23:10811. https://doi.org/10.3390/ijms23181081
Bou-Fakhredin R, De Franceschi L, Motta I et al (2022) Redox balance in β-thalassemia and sickle cell disease: a love and hate relationship. Antioxidants (Basel) 11:967. https://doi.org/10.3390/antiox11050967
Kato GJ, Piel FB, Reid CD et al (2018) Sickle cell disease. Nat Rev Dis Prim 4:1–22. https://doi.org/10.1038/nrdp.2018.10
Iolascon A, Andolfo I, Russo R (2020) Congenital dyserythropoietic anemias. Blood 136:1274–1283. https://doi.org/10.1182/blood.2019000948
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:453–462. https://doi.org/10.1016/j.cub.2014.03.034
Fibach E, Rachmilewitz E (2008) The role of oxidative stress in hemolytic anemia. Curr Mol Med 8:609–619. https://doi.org/10.2174/156652408786241384
Massaccesi L, Galliera E, Romanelli MMC (2020) Erythrocytes as markers of oxidative stress related pathologies. Mech Ageing Dev 191:111333. https://doi.org/10.1016/j.mad.2020.111333
Fujii J, Homma T, Kobayashi S et al (2021) Erythrocytes as a preferential target of oxidative stress in blood. Free Radic Res 55:562–580. https://doi.org/10.1080/10715762.2021.1873318
Voskou S, Aslan M, Fanis P et al (2015) Oxidative stress in β-thalassaemia and sickle cell disease. Redox Biol 6:226–239. https://doi.org/10.1016/j.redox.2015.07.018
Rifkind JM, Mohanty JG, Nagababu E (2015) The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol 5:1–7. https://doi.org/10.3389/fphys.2014.00500
Kingsley PD, Greenfest-Allen E, Frame JM et al (2013) Ontogeny of erythroid gene expression. Blood 121:e5–e13. https://doi.org/10.1182/blood-2012-04-422394
Da Cunha AF, Brugnerotto AF, Duarte AS et al (2010) Global gene expression reveals a set of new genes involved in the modification of cells during erythroid differentiation. Cell Prolif 43:297–309. https://doi.org/10.1111/j.1365-2184.2010.00679.x
Möller MN, Orrico F, Villar SF et al (2023) Oxidants and antioxidants in the redox biochemistry of human red blood cells. ACS Omega 8:147–168. https://doi.org/10.1021/acsomega.2c06768
Neumann CA, Krause DS, Carman CV et al (2003) Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424:561–565. https://doi.org/10.1038/nature01819
Lee T, Kim S, Yu S et al (2003) Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101:5033–5038. https://doi.org/10.1182/blood-2002-08-2548.Supported
Han YH, Kim SU, Kwon TH et al (2012) Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem Biophys Res Commun 426:427–432. https://doi.org/10.1016/j.bbrc.2012.08.113
Manevich Y, Sweitzer T, Pak JH et al (2002) 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99:11599–11604. https://doi.org/10.1073/pnas.182384499
Phelan SA, Wang X, Wallbrandt P et al (2003) Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med 35:1110–1120. https://doi.org/10.1016/S0891-5849(03)00462-3
Kümin A, Huber C, Rülicke T et al (2006) Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis. Am J Pathol 169:1194–1205. https://doi.org/10.2353/ajpath.2006.060119
Walsh B, Pearl A, Suchy S et al (2013) Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep 14:275–284. https://doi.org/10.1179/135100009X12525712409652
Wang X, Phelan SA, Forsman-semb K et al (2003) Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress *. J Biol Chem 278:25179–25190. https://doi.org/10.1074/jbc.M302706200
Sue GR, Ho ZC, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38:1543–1552. https://doi.org/10.1016/j.freeradbiomed.2005.02.026
Zhang B, Wang Y, Su Y (2009) Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett 286:154–160. https://doi.org/10.1016/j.canlet.2009.04.043
Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680. https://doi.org/10.1038/nrd2222
Oliveira MA, Discola KF, Alves SV et al (2010) Insights into the specificity of thioredoxin reductase-thioredoxin interactions. A structural and functional investigation of the yeast thioredoxin system. Biochemistry 49:3317–3326. https://doi.org/10.1021/bi901962p
Poole LB, Hall A, Nelson KJ (2012) Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol 1–20. https://doi.org/10.1002/0471140856.tx0709s49
Hofmann B, Hecht H, Flohé L (2002) Peroxiredoxins. Biol Chem 383:347–364. https://doi.org/10.1515/BC.2002.040
Copley SD, Novak WRP, Babbitt PC (2004) Divergence of function in the thioredoxin fold suprafamily: evidence for evolution of peroxiredoxins from a thioredoxin-like ancestor. Biochemistry 3:13981–13995. https://doi.org/10.1021/bi048947r
Trivelli X, Krimm I, Ebel C et al (2003) Characterization of the yeast peroxiredoxin Ahp1 in its reduced active and overoxidized inactive forms using NMR. Biochemistry 42:14139–14149. https://doi.org/10.1021/bi035551r
Wood ZA (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science (80) 300:650–3. https://doi.org/10.1126/science.1080405
Soito L, Williamson C, Knutson ST et al (2011) PREX: PeroxiRedoxin classification indEX, a database of subfamily assignments across the diverse peroxiredoxin family. Nucleic Acids Res 39:332–337. https://doi.org/10.1093/nar/gkq1060
Barranco-medina S, Lázaro J, Dietz K (2009) The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett 583:1809–1816. https://doi.org/10.1016/j.febslet.2009.05.029
Poole LB (2007) The catalytic mechanism of peroxiredoxins. Subcell Biochem 44:61–81. https://doi.org/10.1007/978-1-4020-6051-9_4
Rhee SG, Woo HA, Kil IS, Bae SH (2012) Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 287:4403–4410. https://doi.org/10.1074/jbc.R111.283432
Monteiro G, Horta BB, Pimenta DC et al (2007) Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc Natl Acad Sci USA 104:4886–4891. https://doi.org/10.1073/pnas.0700481104
Rhee SG, Woo HA (2011) Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxidants Redox Signal 15:781–794. https://doi.org/10.1089/ars.2010.3393
Jang HH, Lee KO, Chi YH et al (2004) Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117:625–635. https://doi.org/10.1016/j.cell.2004.05.002
Vivancos AP, Castillo EA, Biteau B et al (2005) A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci U S A 102:1–6. https://doi.org/10.1073/pnas.0503251102
Hopkins BL, Neumann CA (2019) Redoxins as gatekeepers of the transcriptional oxidative stress response. Redox Biol 21:2213–2317. https://doi.org/10.1016/j.redox.2019.101104
Demasi APD, Pereira GAG, Netto LES (2001) Cytosolic thioredoxin peroxidase I is essential for the antioxidant defense of yeast with dysfunctional mitochondria. FEBS Lett 509:430–434. https://doi.org/10.1016/s0014-5793(01)03215-x
Hall A, Karplus PA, Poole LB (2009) Typical 2-Cys peroxiredoxins – structures, mechanisms and functions. FEBS J 276:2469–2477. https://doi.org/10.1111/j.1742-4658.2009.06985.x
Santos MC, Breyer CA, Schultz L et al (2017) Saccharomyces cerevisiae peroxiredoxins in biological processes: antioxidant defense, signal transduction, circadian rhythm, and more. In: Lucas C, Pais C (eds) Old Yeasts - New Questions. InTech. 119–37
Fujii J, Ikeda Y (2002) Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep 7:123–130. https://doi.org/10.1179/135100002125000352
Kumsta C, Jakob U (2009) Redox-regulated chaperones. Biochemistry 48:4666–4676. https://doi.org/10.1021/bi9003556
Mu ZM, Yin XY, Prochownik EV (2002) Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem 277:43175–43184. https://doi.org/10.1074/jbc.M206066200
Graves JA, Metukuri M, Scott D et al (2009) Regulation of reactive oxygen species homeostasis by peroxiredoxins and c-Myc. J Biol Chem 284:6520–6529. https://doi.org/10.1074/jbc.M807564200
Hansen JM, Moriarty-Craige S, Jones DP (2007) Nuclear and cytoplasmic peroxiredoxin-1 differentially regulate NF-κB activities. Free Radic Biol Med 43:282–288. https://doi.org/10.1016/j.freeradbiomed.2007.04.029
Wang X, He S, Sun J et al (2010) Selective association of peroxiredoxin 1 with genomic DNA and COX-2 upstream promoter elements in estrogen receptor negative breast cancer cells. Mol Biol Cell 21:2987–2995. https://doi.org/10.1091/mbc.E10
Park S, Yu X, Ip C et al (2007) Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res 67:9294–9304. https://doi.org/10.1158/0008-5472.CAN-07-0651
Rhee SG, Kil IS (2017) Multiple functions and regulation of mammalian peroxiredoxins. Annu Rev Biochem 86:749–775. https://doi.org/10.1146/annurev-biochem-060815-014431
Neumann CA, Cao J, Manevich Y (2009) Peroxiredoxin 1 and its role in cell signaling. Cell Cycle 8:4072–4078. https://doi.org/10.4161/cc.8.24.10242
Chang TS, Jeong W, Choi SY et al (2002) Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem 277:25370–25376. https://doi.org/10.1074/jbc.M110432200
Chae HZ, Uhm TB, Rhee SG (1994) Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc Natl Acad Sci U S A 91:7022–7026. https://doi.org/10.1073/pnas.91.15.7022
Yang KS, Kang SW, Woo HA et al (2002) Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem 277:38029–38036. https://doi.org/10.1074/jbc.M206626200
Jung CL, Choi HI, Yu SP et al (2008) Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J Biol Chem 283:28873–28880. https://doi.org/10.1074/jbc.M804087200
Rhee SG, Jeong W, Chang TS, Woo HA (2007) Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int 72:3–8. https://doi.org/10.1038/sj.ki.5002380
Sadowska-Bartosz I, Bartosz G (2023) Peroxiredoxin 2: an important element of the antioxidant defense of the erythrocyte. Antioxidants 12:1–30. https://doi.org/10.3390/antiox12051012
Lei XG, Zhu JH, Cheng WH et al (2015) Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev 96:307–364. https://doi.org/10.1152/physrev.00010.2014
Woo HA, Yim SH, Shin DH et al (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140:517–528. https://doi.org/10.1016/j.cell.2010.01.009
Cao J, Schulte J, Knight A et al (2009) Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J 28:1505–1517. https://doi.org/10.1038/emboj.2009.101
Ding C, Fan X, Wu G (2017) Peroxiredoxin 1 – an antioxidant enzyme in cancer. J Cell Mol Med 21:193–202. https://doi.org/10.1111/jcmm.12955
Stambolic V, Suzuki A, De la Pompa JL et al (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39. https://doi.org/10.1016/S0092-8674(00)81780-8
Lee SR, Yang KS, Kwon J et al (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277:20336–20342. https://doi.org/10.1074/jbc.M111899200
Huo YY, Li G, Duan RF et al (2008) PTEN deletion leads to deregulation of antioxidants and increased oxidative damage in mouse embryonic fibroblasts. Free Radic Biol Med 44:1578–1591. https://doi.org/10.1016/j.freeradbiomed.2008.01.013
Nassour H, Wang Z, Saad A et al (2016) Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression. Sci Rep 6:1–17. https://doi.org/10.1038/srep29389
Xanthoudakis S, Miao G, Wang F et al (1992) Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J 11:3323–3335. https://doi.org/10.1002/j.1460-2075.1992.tb05411.x
Ema M (1999) Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J 18:1905–1914. https://doi.org/10.1093/emboj/18.7.1905
Tell G, Pellizzari L, Cimarosti D et al (1998) Ref-1 controls Pax-8 DNA-binding activity. Biochem Biophys Res Commun 252:178–183. https://doi.org/10.1006/bbrc.1998.9548
Gaiddon C, Moorthy NC, Prives C (1999) Ref-1 regulates the transactivation and pro-apoptotic functions of p53 in vivo. EMBO J 18:5609–5621. https://doi.org/10.1093/emboj/18.20.5609
Bapat A, Fishel ML, Kelley MR (2009) Going ape as an approach to cancer therapeutics. Antioxidants Redox Signal 11:651–667. https://doi.org/10.1089/ars.2008.2218
De Larco JE, Wuertz BRK, Rosner KA et al (2001) A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 158:639–646. https://doi.org/10.1016/S0002-9440(10)64005-9
Chen WT, Ebelt ND, Stracker TH et al (2015) ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife 4:e07270. https://doi.org/10.7554/eLife.07270
Romanello KS, Teixeira KKL, Silva JPMO et al (2018) Global analysis of erythroid cells redox status reveals the involvement of Prdx1 and Prdx2 in the severity of beta thalassemia. PLoS ONE 13:1–19. https://doi.org/10.1371/journal.pone.0208316
Maia de Oliveira da Silva JP, Brugnerotto AF, S. Romanello K et al (2019) Global gene expression reveals an increase of HMGB1 and APEX1 proteins and their involvement in oxidative stress, apoptosis and inflammation pathways among beta-thalassaemia intermedia and major phenotypes. Br J Haematol 186:608–619. https://doi.org/10.1111/bjh.16062
Bernardo VS, Torres FF, Paula CP de et al (2022) Potential cytoprotective and regulatory effects of ergothioneine on gene expression of proteins involved in erythroid adaptation mechanisms and redox pathways in. Genes (Basel) 13:2368. https://doi.org/10.3390/genes13122368
Menon V, Ghaffari S (2021) Erythroid enucleation: a gateway into a “bloody” world. Exp Hematol 95:13–22. https://doi.org/10.1016/j.exphem.2021.01.001
Menon V, Ghaffari S (2018) Transcription factors FOXO in the regulation of homeostatic hematopoiesis. Curr Opin Hematol 25:290–298. https://doi.org/10.1097/MOH.0000000000000441
Hopkins BL, Nadler M, Skoko JJ et al (2018) A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7. Antioxidants Redox Signal 28:62–77. https://doi.org/10.1089/ars.2016.6871
Troussicot L, Burmann BM, Molin M (2021) Structural determinants of multimerization and dissociation in 2-Cys peroxiredoxin chaperone function. Structure 29:640–654. https://doi.org/10.1016/j.str.2021.04.007
Jarvis RM, Hughes SM, Ledgerwood EC (2012) Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic Biol Med 53:1522–1530. https://doi.org/10.1016/j.freeradbiomed.2012.08.001
Gertz M, Fischer F, Leipelt M et al (2009) Identification of Peroxiredoxin 1 as a novel interaction partner for the lifespan regulator protein p66Shc. Aging (Albany NY) 1:254–265. https://doi.org/10.18632/aging.100017
Kim SY, Kim TJ, Lee K-Y (2008) A novel function of peroxiredoxin 1 Prx-1 in apoptosis signal-regulating kinase 1 ASK1. FEBS Lett 582(582):1913–1918. https://doi.org/10.1016/j.febslet.2008.05.015
Saitoh M, Nishitoh H, Fujii M et al (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606. https://doi.org/10.1039/DT9890001143
Kim YJ, Lee WS, Ip C et al (2006) Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res 66:7136–7142. https://doi.org/10.1158/0008-5472.CAN-05-4446
Ishii T, Warabi E, Yanagawa T (2012) Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr 50:91–105. https://doi.org/10.3164/jcbn.11-109
Finetti F, Savino MT, Baldari CT (2009) Positive and negative regulation of antigen receptor signaling by the Shc family of protein adapters. Immunol Rev 232:115–134. https://doi.org/10.1111/j.1600-065X.2009.00826.x
Giorgio M, Migliaccio E, Orsini F et al (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233. https://doi.org/10.1016/j.cell.2005.05.011
Centis F, Tabellini L, Lucarelli G et al (2000) The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with β-thalassemia major. Blood 96:3624–3629. https://doi.org/10.1182/blood.V96.10.3624
Pootrakul P, Sirankapracha P, Hemsorach S et al (2000) A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood 96:2606–2612. https://doi.org/10.1182/blood.V96.7.2606
Mathias LA, Fisher TC, Zeng L et al (2000) Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol 28:1343–1353. https://doi.org/10.1016/s0301-472x(00)00555-5
Tamary BH, Shalev H, Luria D et al (1996) Clinical Features and studies of erythropoiesis in Israeli Bedouins with congenital dyserythropoietic anemia type I. Blood 87:1763–1770. https://doi.org/10.1182/blood.V87.5.1763.1763
Yehia L, Niazi F, Ni Y et al (2015) Germline heterozygous variants in SEC23B are associated with Cowden syndrome and enriched in apparently sporadic thyroid cancer. Am J Hum Genet 97:661–676. https://doi.org/10.1016/j.ajhg.2015.10.001
Kim E, Kim Y, Yang JY, Jang HH (2022) Prx1 regulates thapsigargin-mediated UPR activation and apoptosis. Genes (Basel) 13:2033. https://doi.org/10.3390/genes13112033
Schröder E, Littlechild JA, Lebedev AA et al (2000) Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7 Å resolution. Structure 8:605–615. https://doi.org/10.1016/S0969-2126(00)00147-7
Plishker GA, Chevalier D, Seinsoth L, Moore RB (1992) Calcium-activated potassium transport and high molecular weight forms of calpromotin. J Biol Chem 267:21839–21843. https://doi.org/10.1016/s0021-9258(19)36688-8
Moore RB, Mankad MV, Shriver SK et al (1991) Reconstitution of Ca2+-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem 266:18964–18968. https://doi.org/10.1016/s0021-9258(18)55157-7
Harris JR (1969) Some negative contrast staining features of a protein from erythrocite ghosts. J Mol Biol 46:329–335. https://doi.org/10.1016/0022-2836(69)90425-2
Harris JR, Naeem I (1981) Further studies on the characterization of cylindrin and torin, two extrinsic proteins of the erythrocyte membrane. BBA - Protein Struct 670:285–290. https://doi.org/10.1016/0005-2795(81)90021-0
Matte A, Bertoldi M, Mohandas N et al (2013) Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic Biol Med 55:27–35. https://doi.org/10.1016/j.freeradbiomed.2012.10.543
Chu H, Breite A, Ciraolo P et al (2008) Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties. Blood 111:932–938. https://doi.org/10.1182/blood-2007-07-100180
Cha MK, Yun CH, Kim IH (2000) Interaction of human thiol-specific antioxidant protein 1 with erythrocyte plasma membrane. Biochemistry 39:6944–6950. https://doi.org/10.1021/bi000034j
Cha MK, Kim IH (1996) Purification and characterization of thiol-specific antioxidant protein from human liver: a Mer5-like human isoenzyme. J Biochem Mol Biol 29:236–240
Mattè A, Federti E, Tibaldi E et al (2021) Tyrosine phosphorylation modulates peroxiredoxin-2 activity in normal and diseased red cells. Antioxidants 10:1–16. https://doi.org/10.3390/antiox10020206
Rocha S, Costa E, Coimbra S et al (2009) Linkage of cytosolic peroxiredoxin 2 to erythrocyte membrane imposed by hydrogen peroxide-induced oxidative stress. Blood Cells Mol Dis 43:68–73. https://doi.org/10.1016/j.bcmd.2009.03.002
Biondani A, Turrini F, Carta F et al (2008) Heat-shock protein-27, -70 and peroxiredoxin-II show molecular chaperone function in sickle red cells: Evidence from transgenic sickle cell mouse model. Proteomics Clin Appl 2:706–719. https://doi.org/10.1002/prca.200780058
Rocha S, Vitorino RMP, Lemos-Amado FM et al (2008) Presence of cytosolic peroxiredoxin 2 in the erythrocyte membrane of patients with hereditary spherocytosis. Blood Cells, Mol Dis 41:5–9. https://doi.org/10.1016/j.bcmd.2008.02.008
Moore RB, Shriver SK, Jenkins LD et al (1997) Calpromotin, a cytoplasmic protein, is associated with the formation of dense cells in sickle cell anemia. Am J Hematol 56:100–106. https://doi.org/10.1002/(SICI)1096-8652(199710)56:2%3c100::AID-AJH5%3e3.0.CO;2-2
Matte A, Low PS, Turrini F et al (2010) Peroxiredoxin-2 expression is increased in β-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med 49:457–466. https://doi.org/10.1016/j.freeradbiomed.2010.05.003
Bayer SB, Low FM, Hampton MB, Winterbourn CC (2016) Interactions between peroxiredoxin 2, hemichrome and the erythrocyte membrane. Free Radic Res 50:1329–1339. https://doi.org/10.1080/10715762.2016.1241995
Ma Q, An L, Tian H et al (2019) Interactions between human hemoglobin subunits and peroxiredoxin 2. Front Biosci - Landmark Ed 24:1085–1096. https://doi.org/10.2741/4770
Basu A, Chakrabarti A (2015) Hemoglobin interacting proteins and implications of spectrin hemoglobin interaction. J Proteomics 128:469–475. https://doi.org/10.1016/j.jprot.2015.06.014
Rinalducci S, D’Amici GM, Blasi B, Zolla L (2011) Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie 93:845–853. https://doi.org/10.1016/j.biochi.2011.02.005
Matte A, De Franceschi L (2019) Oxidation and erythropoiesis. Curr Opin Hematol 26:145–151. https://doi.org/10.1097/MOH.0000000000000495
Johnson RM, Ho Y-S, Yu D-Y et al (2010) The effect of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1 and catalase on erythrocyte oxidative metabolism. Free Radic Biol Med 48:1–19. https://doi.org/10.1016/j.freeradbiomed.2009.11.021.The
De FL, Bertoldi M, De FL et al (2011) Oxidative stress modulates heme synthesis and induces peroxiredoxin-2 as a novel cytoprotective response in β-thalassemic erythropoiesis. Haematologica 96:1595–1604. https://doi.org/10.3324/haematol.2011.043612
Matte A, De Falco L, Iolascon A et al (2015) The interplay between peroxiredoxin-2 and nuclear factor-erythroid 2 is important in limiting oxidative mediated dysfunction in β-thalassemic erythropoiesis. Antioxidants Redox Signal 23:1284–1297. https://doi.org/10.1089/ars.2014.6237
Emberesh M, Giger Seu K, Emberesh S et al (2018) Peroxiredoxin II (PRDX2) is a novel candidate gene for congenital dyserythropoietic anemia. Blood 132:3605–3605. https://doi.org/10.1182/blood-2018-99-120056
Ghashghaeinia M, Toulany M, Saki M et al (2012) Roles of the NFκB and glutathione pathways in mature human erythrocytes. Cell Mol Biol Lett 17:11–20. https://doi.org/10.2478/s11658-011-0032-x
Cho C, Yoon HJ, Kim JY et al (2014) Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. PNAS 111:12043–12048. https://doi.org/10.1073/pnas.1401100111
O-Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469:498–504. https://doi.org/10.1038/nature09702
Urbinati F, Madigan C, Malik P (2006) Pathophysiology and therapy for haemoglobinopathies. Part II: Thalassaemias. Expert Rev Mol Med 8:1–26. https://doi.org/10.1017/S1462399406010805
Dunn LL, Rahmanto YS, Richardson DR (2007) Iron uptake and metabolism in the new millennium. Trends Cell Biol 17:93–100. https://doi.org/10.1016/j.tcb.2006.12.003
Muckenthaler MU, Rivella S, Hentze MW, Galy B (2017) A red carpet for iron metabolism. Cell 168:344–361. https://doi.org/10.1016/j.cell.2016.12.034
Sobotta MC, Liou W, Stöcker S et al (2015) Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11:64–70. https://doi.org/10.1038/nchembio.1695
Matte A, De Falco L, Federti E et al (2018) Peroxiredoxin-2: a novel regulator of iron homeostasis in ineffective erythropoiesis
Talwar D, Messens J, Dick TP (2020) A role for annexin A2 in scaffolding the peroxiredoxin 2–STAT3 redox relay complex. Nat Commun 11:1–11. https://doi.org/10.1038/s41467-020-18324-9
Mo Y, Feinstein SI, Manevich Y et al (2003) 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless. FEBSLetters 555:192–198. https://doi.org/10.1016/S0014-5793(03)01199-2
Fisher AB (2018) The phospholipase A2 activity of peroxiredoxin 6. J Lipid Res 59:1132–1147. https://doi.org/10.1194/jlr.R082578
Cho C, Lee JS, Gladwin MT, Rhee SG (2010) Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid Redox Signal 13:1–11
Kubo E, Chhunchha B, Singh P et al (2017) Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep 7:1–17. https://doi.org/10.1038/s41598-017-14520-8
Panda H, Keleku-Lukwete N, Kuga A et al (2019) Dietary supplementation with sulforaphane attenuates liver damage and heme overload in a sickle cell disease murine model. Exp Hematol 77:51-60.e1. https://doi.org/10.1016/j.exphem.2019.08.001
Lu B, Chen X, bing, Hong Y cai, et al (2019) Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol Sin 40:1334–1342. https://doi.org/10.1038/s41401-019-0233-9
Saki N, Abroun S, Salari F et al (2015) Molecular aspects of bone resorption in β-thalassemia major. Cell J 17:193–200
Stuhlmeier KM, Kao JJ, Wallbrandt P et al (2003) Antioxidant protein 2 prevents methemoglobin formation in erythrocyte hemolysates. Eur J Biochem 270:334–341. https://doi.org/10.1046/j.1432-1033.2003.03393.x
López-Grueso MJ, Lagal DJ, García-Jiménez ÁF, et al (2020) Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells. Redox Biol 37. https://doi.org/10.1016/j.redox.2020.101737
Jia W, Dong C, Li B (2023) Anti-oxidant and pro-oxidant effects of peroxiredoxin 6: a potential target in respiratory diseases. 1–14
Acknowledgements
We thank the Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com) for the image used to production of the figures presented in this article.
Funding
This work was supported by FAPESP (2011/50358-3) and CAPES. Author JPMOS has received research support from CAPES.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception and design. Carla Peres de Paula wrote the first draft of the manuscript. CPP, KSR, JPMOS, VSB, and FFT reviewed the literature. VSB, FFT, AFC, and DGHS critically revised the work. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors concur with the submission.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Paula, C.P., de Oliveira da Silva, J.P.M., Romanello, K.S. et al. Peroxiredoxins in erythrocytes: far beyond the antioxidant role. J Mol Med 101, 1335–1353 (2023). https://doi.org/10.1007/s00109-023-02368-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02368-7