Abstract
Purpose
The purpose of this study was to investigate the predictive value of changes in serum uric acid (SUA), the ratio of serum uric acid to serum creatinine (SUA/SCr), and serum gamma-glutamyltransferase (GGT) from before to after therapy in patients with locally advanced rectal cancer (LARC).
Methods
Data from 114 LARC patients from January 2016 to December 2021 were included in this retrospective study. All patients received neoadjuvant chemoradiotherapy (nCRT) and total mesorectal excision (TME). The change in SUA was calculated as a ratio: (SUA level after nCRT–SUA level before nCRT)/SUA level before nCRT. The change ratios of SUA/SCr and GGT were calculated in the same way. The efficacy of nCRT was evaluated by magnetic resonance (MR) and postoperative pathological response. A nonlinear model was used to evaluate whether the change ratios of SUA, SUA/SCr, and GGT were associated with the efficacy of nCRT. The predictive power of the change ratios of SUA, SUA/SCr, and GGT was assessed by receiver operating characteristic (ROC) curves. Univariate and multivariate Cox regression analyses were employed to measure the associations between disease-free survival (DFS) and other predictive indicators. The Kaplan–Meier method was used to further compare DFS between groups.
Results
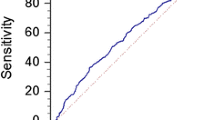
The nonlinear model indicated that the change ratios of SUA, SUA/SCr, and GGT were associated with the efficacy of nCRT. The change ratios of SUA, SUA/SCr, and GGT were used to predict the area under the ROC curve of efficacy for nCRT (0.95, 0.91–0.99), which was better than the prediction by the change ratio of SUA (0.94, 0.89–0.99), SUA/SCr (0.90, 0.84–0.96), or GGT alone (0.86, 0.79–0.93; p < 0.05). The optimal cut-off values of SUA, SUA/SCr, and GGT change were 0.02, 0.01, and 0.04, respectively. The Kaplan–Meier method indicated that patients with SUA, SUA/SCr, or GGT changes greater than the cut-off values had shorter DFS (p < 0.05).
Conclusion
Change ratios of SUA, SUA/SCr, or GGT greater than the cut-off values implied a risk of poor pathological response after nCRT and shorter DFS in LARC patients.






Similar content being viewed by others
References
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D et al (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16(8):979–989
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30(16):1926–1933
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Ballonoff A, Kavanagh B, McCarter M, Kane M, Pearlman N, Nash R et al (2008) Preoperative capecitabine and accelerated intensity-modulated radiotherapy in locally advanced rectal cancer: a phase II trial. Am J Clin Oncol 31(3):264–270
Gérard J, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin M et al (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‑4 rectal cancers: results of FFCD 9203. J Clin Oncol 24(28):4620–4625
Bosset J, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Bosset J, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A et al (2005) Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol 23(24):5620–5627
Bosset J, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P et al (2004) Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC radiotherapy group. Eur J Cancer 40(2):219–224
Rades D, Kuhn H, Schultze J, Homann N, Brandenburg B, Schulte R et al (2008) Prognostic factors affecting locally recurrent rectal cancer and clinical significance of hemoglobin. Int J Radiat Oncol Biol Phys 70(4):1087–1093
Matsuyama T, Yamauchi S, Masuda T, Kikuchi A, Tokunaga M, Sugihara K et al (2021) Treatment and subsequent prognosis in locally recurrent rectal cancer: a multicenter retrospective study of 498 patients. Int J Colorectal Dis 36(6):1243–1250
Guraya S (2019) Pattern, stage, and time of recurrent colorectal cancer after curative surgery. Clin Colorectal Cancer 18(2):e223–e8
Westberg K, Palmer G, Hjern F, Johansson H, Holm T, Martling A (2018) Management and prognosis of locally recurrent rectal cancer—a national population-based study. Eur J Surg Oncol 44(1):100–107
Baird D, Kontovounisios C, Simillis C, Pellino G, Rasheed S, Tekkis P (2020) Factors associated with metachronous metastases and survival in locally advanced and recurrent rectal cancer. BJS Open 4(6):1172–1179
Jörgren F, Johansson R, Damber L, Lindmark G (2010) Risk factors of rectal cancer local recurrence: population-based survey and validation of the Swedish rectal cancer registry. Colorectal Dis 12(10):977–986
Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M et al (2018) MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 287(3):833–843
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub M (2019) MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics 39(2):367–387
Leijssen L, Dinaux A, Amri R, Taylor M, Deshpande V, Bordeianou L et al (2019) Impact of intramural and extramural vascular invasion on stage II-III colon cancer outcomes. J Surg Oncol 119(6):749–757
Kim Y, Kim C, Kim J, Kim J, Ro J, Lee J et al (2022) Clinical implication of perineural and lymphovascular invasion in rectal cancerpatients who underwent surgery after preoperative chemoradiotherapy. Dis Colon Rectum 65(11):1325–1334. https://doi.org/10.1097/DCR.0000000000002219
Sun Q, Liu T, Liu P, Luo J, Zhang N, Lu K et al (2019) Perineural and lymphovascular invasion predicts for poor prognosis in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery. J Cancer 10(10):2243–2249
Yang C, Lin L, Lin Y, Tian Y, Lin C, Sheu M et al (2019) Higher nuclear EGFR expression is a better predictor of survival in rectal cancer patients following neoadjuvant chemoradiotherapy than cytoplasmic EGFR expression. Oncol Lett 17(2):1551–1558
Meng X, Wang R, Huang Z, Zhang J, Feng R, Xu X et al (2014) Human epidermal growth factor receptor‑2 expression in locally advanced rectal cancer: association with response to neoadjuvant therapy and prognosis. Cancer Sci 105(7):818–824
El Otmani I, El Agy F, El Baradai S, Bouguenouch L, Lahmidani N, El Abkari M et al (2020) Analysis of molecular pretreated tumor profiles as predictive biomarkers of therapeutic response and survival outcomes after neoadjuvant therapy for rectal cancer in Moroccan population. Dis Markers 2020:8459303
Ye S, Cheng Y, Zhang L, Zou Y, Chen P, Deng Y et al (2020) Association of mismatch repair status with survival and response to neoadjuvant chemo(radio)therapy in rectal cancer. NPJ Precis Oncol 4:26
de Rosa N, Rodriguez-Bigas M, Chang G, Veerapong J, Borras E, Krishnan S et al (2016) DNA mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol 34(25):3039–3046
Xing Y, Qin F, Zhai Y, Yang J, Yan Y, Li D et al (2022) Association of clinical features of colorectal cancer with circulating tumor cells and systemic inflammatory markers. Dis Markers 2022:5105599
Pietrzyk Ł (2016) Biomarkers discovery for colorectal cancer: a review on tumor endothelial markers as perspective candidates. Dis Markers 2016:4912405
Walkiewicz K, Strzelczyk J, Waniczek D, Biernacki K, Muc-Wierzgoń M, Copija A et al (2019) Adamalysines as biomarkers and a potential target of therapy in colorectal cancer patients: preliminary results. Dis Markers 2019:5035234
Sung S, Son S, Park E, Kay C (2017) Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS ONE 12(3):e173955
Bottarelli L, De’ Angelis G, Azzoni C, Di Mario F, De’ Angelis N, Leandro G et al (2018) Potential predictive biomarkers in locally advanced rectal cancer treated with preoperative chemo-radiotherapy. Acta Biomed 89:102–106
Rodrigues D, Simões J, Teixeira L, Aires F, Fernandes C, Rey C et al (2021) Baseline anaemia increases locally advanced rectal cancer mortality in older patients undergoing preoperative chemoradiation. Support Care Cancer 29(3):1403–1411
Sakin A, Sahin S, Karyagar S, Karyagar S, Atci M, Akboru M et al (2022) The predictive value of baseline volumetric PET/CT parameters on treatment response and prognosis in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. J Gastrointest Cancer 53(2):341–347. https://doi.org/10.1007/s12029-021-00608-y
Pang X, Wang F, Zhang Q, Li Y, Huang R, Yin X et al (2021) A pipeline for predicting the treatment response of neoadjuvant chemoradiotherapy for locally advanced rectal cancer using single MRI modality: combining deep segmentation network and radiomics analysis based on “suspicious region”. Front Oncol 11:711747
Nikolouzakis T, Vakonaki E, Stivaktakis P, Alegakis A, Berdiaki A, Razos N et al (2021) Novel prognostic biomarkers in metastatic and locally advanced colorectal cancer: micronuclei frequency and telomerase activity in peripheral blood lymphocytes. Front Oncol 11:683605
Dovell F, Boffetta P (2018) Serum uric acid and cancer mortality and incidence: a systematic review and meta-analysis. Eur J Cancer Prev 27(4):399–405
Huang C, Huang J, Mi N, Lin Y, He Q, Lu Y et al (2020) Associations between serum uric acid and hepatobiliary-pancreatic cancer: a cohort study. World J Gastroenterol 26(44):7061–7075
Xiao Y, Yang H, Lu J, Li D, Xu C, Risch H (2019) Serum gamma-glutamyltransferase and the overall survival of metastatic pancreatic cancer. BMC Cancer 19(1):1020
Taylor F, Swift R, Blomqvist L, Brown G (2008) A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol 191(6):1827–1835
Beets-Tan R, Lambregts D, Maas M, Bipat S, Barbaro B, Curvo-Semedo L et al (2018) Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting. Eur Radiol 28(4):1465–1475
Beets-Tan R, Lambregts D, Maas M, Bipat S, Barbaro B, Caseiro-Alves F et al (2013) Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting. Eur Radiol 23(9):2522–2531
Patel U, Blomqvist L, Taylor F, George C, Guthrie A, Bees N et al (2012) MRI after treatment of locally advanced rectal cancer: how to report tumor response—the MERCURY experience. Ajr Am J Roentgenol 199(4):W486–95
Patel U, Taylor F, Blomqvist L, George C, Evans H, Tekkis P et al (2011) Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29(28):3753–3760
Bates D, Homsi M, Chang K, Lalwani N, Horvat N, Sheedy S (2022) MRI for rectal cancer: staging, mrCRM, EMVI, lymph node staging and post-treatment response. Clin Colorectal Cancer 21(1):10–18
Inoue A, Sheedy S, Heiken J, Mohammadinejad P, Graham R, Lee H et al (2021) MRI-detected extramural venous invasion of rectal cancer: multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging 12(1):110
Vecchio F, Valentini V, Minsky B, Padula G, Venkatraman E, Balducci M et al (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62(3):752–760
Huh J, Kim H, Kim S, Park Y, Cho Y, Yun S et al (2019) Tumor regression grade as a clinically useful outcome predictor in patients with rectal cancer after preoperative chemoradiotherapy. Surgery 165(3):579–585
Trakarnsanga A, Gönen M, Shia J, Nash G, Temple L, Guillem J et al (2014) Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. JNCI J Natl Cancer Inst 106(10):dju248. https://doi.org/10.1093/jnci/dju248
Hofheinz R, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann J et al (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13(6):579–588
Roh M, Colangelo L, O’Connell M, Yothers G, Deutsch M, Allegra C et al (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R‑03. J Clin Oncol 27(31):5124–5130
Fini M, Elias A, Johnson R, Wright R (2012) Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Trans Med 1(1):16
Xie Y, Xu P, Liu K, Lin S, Wang M, Tian T et al (2019) Hyperuricemia and gout are associated with cancer incidence and mortality: a meta-analysis based on cohort studies. J Cell Physiol 234(8):14364–14376
Dai X, He Q, Jing Z, Yuan J (2020) Serum uric acid levels and risk of kidney cancer incidence and mortality: a prospective cohort study. Cancer Med 9(15):5655–5661
Mi N, Huang J, Huang C, Lin Y, He Q, Wang H et al (2022) High serum uric acid may associate with the increased risk of colorectal cancer in females: a prospective cohort study. Int J Cancer 150(2):263–272
Yue C, Feng P, Yao Z, Yu X, Lin W, Qian Y et al (2017) High serum uric acid concentration predicts poor survival in patients with breast cancer. Clin Chim Acta 473:160–165
Wu L, Yang W, Zhang Y, Du X, Jin N, Chen W et al (2021) Elevated serum uric acid is associated with poor survival in advanced HCC patients and febuxostat improves prognosis in HCC rats. Front Pharmacol 12:778890
Mao L, Guo C, Zheng S (2018) Elevated urinary 8‑oxo‑7,8‑dihydro-2′-deoxyguanosine and serum uric acid are associated with progression and are prognostic factors of colorectal cancer. Onco Targets Ther 11:5895–5902
Yiu A, Van Hemelrijck M, Garmo H, Holmberg L, Malmström H, Lambe M et al (2017) Circulating uric acid levels and subsequent development of cancer in 493,281 individuals: findings from the AMORIS Study. Oncotarget 8(26):42332–42342
Louthrenoo W, Kasitanon N, Wichainun R, Sukitawut W (2002) Effect of minidose aspirin on renal function and renal uric acid handling in healthy young adults. J Clin Rheumatol 8(6):299–304
Gao X, Curhan G, Forman J, Ascherio A, Choi H (2008) Vitamin C intake and serum uric acid concentration in men. J Rheumatol 35(9):1853–1858
MacFarlane L, Liu C, Solomon D (2015) The effect of initiating pharmacologic insulin on serum uric acid levels in patients with diabetes: a matched cohort analysis. Semin Arthritis Rheum 44(5):592–596
Hisamatsu E, Kawai K, Hinotsu S, Miyanaga N, Shimazui T, Akaza H (2005) Serum creatinine and cholesterol levels of testicular cancer patients in long-term follow up. Int J Urol 12(8):751–756
Schwameis R, Postl M, Bekos C, Hefler L, Reinthaller A, Seebacher V et al (2019) Prognostic value of serum creatine level in patients with vulvar cancer. Sci Rep 9(1):11129
Lafleur J, Hefler-Frischmuth K, Grimm C, Schwameis R, Gensthaler L, Reiser E et al (2018) Prognostic value of serum creatinine levels in patients with epithelial ovarian cancer. Anticancer Res 38(9):5127–5130
Yang M, Zhang Q, Ruan G, Tang M, Zhang X, Song M et al (2021) Association between serum creatinine concentrations and overall survival in patients with colorectal cancer: a multi-center cohort study. Front Oncol 11:710423
das Neves W, Alves C, de Souza Borges A, de Castro G (2021) Serum creatinine as a potential biomarker of skeletal muscle atrophy in non-small cell lung cancer patients. Front Physiol 12:625417
Mazidi M, Katsiki N, Banach M (2021) Α higher ratio of serum uric acid to serum creatinine could predict the risk of total and cause specific mortality- insight from a US national survey. Int J Cardiol 326:189–193
Al-Daghri N, Al-Attas O, Wani K, Sabico S, Alokail M (2017) Serum uric acid to creatinine ratio and risk of metabolic syndrome in saudi type 2 diabetic patients. Sci Rep 7(1):12104
Wang A, Tian X, Wu S, Zuo Y, Chen S, Mo D et al (2021) Metabolic factors mediate the association between serum uric acid to serum creatinine ratio and cardiovascular disease. J Am Heart Assoc 10(23):e23054
Silva N, Gonçalves C, Gonçalves D, Cotta R, da Silva L (2021) Association of uric acid and uric acid to creatinine ratio with chronic kidney disease in hypertensive patients. BMC Nephrol 22(1):311
Mok Y, Son D, Yun Y, Jee S, Samet J (2016) γ‑Glutamyltransferase and cancer risk: the Korean cancer prevention study. Int J Cancer 138(2):311–319
Kunutsor S, Laukkanen J (2017) Gamma-glutamyltransferase and risk of prostate cancer: findings from the KIHD prospective cohort study. Int J Cancer 140(4):818–824
Long Y, Zeng F, Shi J, Tian H, Chen T (2014) Gamma-glutamyltransferase predicts increased risk of mortality: a systematic review and meta-analysis of prospective observational studies. Free Radic Res 48(6):716–728
Cho H, Choi H, Lee K, Cho N (2021) Gamma-glutamyltransferase and the risk of head and neck cancer mortality. J Oral Pathol Med 50(8):803–811
Franzini M, Corti A, Fierabracci V, Pompella A (2014) Helicobacter, gamma-glutamyltransferase and cancer: further intriguing connections. World J Gastroenterol 20(47):18057–18058
Ishiyama Y, Kondo T, Tachibana H, Ishihara H, Fukuda H, Yoshida K et al (2021) Predictive role of γ‑glutamyltransferase in patients receiving nivolumab therapy for metastatic renal cell carcinoma. Int J Clin Oncol 26(3):552–561
Funding
This work was supported by the Basic Scientific Research Operating Expenses of Wenzhou Medical University in 2019 (KYYW201923).
Author information
Authors and Affiliations
Contributions
RX, XZ, and CZ participated in study design, data analysis, and manuscript review. YX and ZS participated in data collection, data analysis, and manuscript writing. All authors reviewed the manuscript and revision and provided final approval for submission.
Corresponding authors
Ethics declarations
Conflict of interest
Z. Shao, Y. Xu, X. Zhang, C. Zou, and R. Xie declare that they have no competing interests.
Ethical standards
The study was carried out in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. The study involving human participants was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. The ethical number is KY2020-R190. A waiver of written consent was requested for this study and the Ethics Committee approved.
Additional information
The authors Zhenyong Shao and Yuyan Xu contributed equally to the manuscript.
Data Availability Statement
The authors will provide the raw data supporting the conclusions of this study without reservation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, Z., Xu, Y., Zhang, X. et al. Changes in serum uric acid, serum uric acid/serum creatinine ratio, and gamma-glutamyltransferase might predict the efficacy of neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Strahlenther Onkol 200, 523–534 (2024). https://doi.org/10.1007/s00066-023-02096-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02096-4