Abstract
Purpose
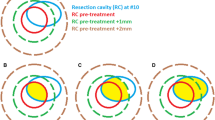
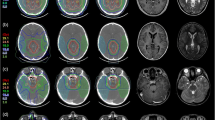
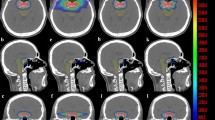
Brainstem radionecrosis is an important issue during the irradiation of tumors of the posterior fossa. The aim of the present study is to analyze postsurgical geometrical variations of tumor bed (TB) and brainstem (BS) and their impact on dosimetry.
Methods
Retrospective collection of data from pediatric patients treated at a single institution. Availability of presurgical magnetic resonance imaging (MRI) was verified; availability of at least two postsurgical MRIs was considered a further inclusion criterion. The following metrics were analyzed: total volume, Dice similarity coefficient (DSC), and Haudsdorff distances (HD).
Results
Fourteen patients were available for the quantification of major postsurgical geometrical variations of TB. DSC, HD max, and HD average values were 0.47 (range: 0.08;0.76), 11.3 mm (7.7;24.5), and 2.6 mm (0.7;6.7) between the first and the second postoperative MRI, respectively. Postsurgical geometrical variations of the BS were also observed. Coverage to the TB was reduced in one patient (D95: −2.9 Gy), while D2 to the BS was increased for the majority of patients. Overall, predictive factors for significant geometrical changes were presurgical gross tumor volume (GTV) > 33 mL, hydrocephaly at diagnosis, Luschka foramen involvement, and younger age (≤ 8 years).
Conclusion
Major volume changes were observed in this cohort, with some dosimetric impact. The use of a recent co-registration MRI is advised. The 2–3 mm HD average observed should be considered in the planning target volume/planning organ at risk volume (PTV/PRV) margin and/or robust optimization planning. Results from wider efforts are needed to verify our findings.



Similar content being viewed by others
Abbreviations
- BS:
-
Brainstem
- CNS:
-
Central nervous system
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- DICOM:
-
Digital Imaging and Communication in Medicine
- DSC:
-
Dice similarity coefficient
- EPTN:
-
European Particle Therapy Network
- GTV:
-
Gross tumor volume
- HD:
-
Hausdorff distance
- IQR:
-
Interquartile range
- LET:
-
Linear energy transfer
- MRI:
-
Magnetic resonance imaging
- NTCP:
-
Normal tissue complication probability
- OAR:
-
Organ at risk
- PF:
-
Posterior fossa
- PRV:
-
Planning organ at risk volume
- PT:
-
Proton therapy
- PTV:
-
Planning target volume
- ROI:
-
Region of interest
- RT:
-
Radiotherapy
- RBE:
-
Relative biological effectiveness
- SOBP:
-
Spread-out Bragg peak
- TB:
-
Tumor bed
- TPS:
-
Treatment planning system
References
Patel AP, Fisher JL, Nichols E, Abd-Allah F, Abdela J, Abdelalim A et al (2019) Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:376–393. https://doi.org/10.1016/S1474-4422(18)30468-X
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC et al (2014) Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev 23:2716–2736. https://doi.org/10.1158/1055-9965.EPI-14-0207
D’Arco F, Khan F, Mankad K, Ganau M, Caro-Dominguez P, Bisdas S (2018) Differential diagnosis of posterior fossa tumours in children: new insights. Pediatr Radiol 48:1955–1963. https://doi.org/10.1007/s00247-018-4224-7
Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ (2013) Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys 87:46–52. https://doi.org/10.1016/j.ijrobp.2013.04.030
Bekelman JE, Schultheiss T, Berrington De Gonzalez A (2013) Subsequent malignancies after photon versus proton radiation therapy. Int J Radiat Oncol Biol Phys 87:10–12. https://doi.org/10.1016/j.ijrobp.2013.05.016
Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS et al (2014) Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol 113:89–94. https://doi.org/10.1016/j.radonc.2014.08.017
Kahalley LS, Ris MD, Grosshans DR, Okcu MF, Paulino AC, Chintagumpala M et al (2016) Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol 34:1043–1049. https://doi.org/10.1200/JCO.2015.62.1383
Pulsifer MB, Duncanson H, Grieco J, Evans C, Tseretopoulos ID, MacDonald S et al (2018) Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys 102:391–398. https://doi.org/10.1016/j.ijrobp.2018.05.069
Ventura LM, Grieco JA, Evans CL, Kuhlthau KA, MacDonald SM, Tarbell NJ et al (2018) Executive functioning, academic skills, and quality of life in pediatric patients with brain tumors post-proton radiation therapy. J Neurooncol 137:119–126. https://doi.org/10.1007/s11060-017-2703-6
Paganetti H (2018) Proton relative biological effectiveness—uncertainties and opportunities. Int J Part Ther 5:2–14. https://doi.org/10.14338/IJPT-18-00011.1
Wedenberg M, Toma-Dasu I (2014) Disregarding RBE variation in treatment plan comparison may lead to bias in favor of proton plans. Med Phys 41:91706. https://doi.org/10.1118/1.4892930
Paganetti H (2014) Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 59:R419–R472. https://doi.org/10.1088/0031-9155/59/22/R419
Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S et al (2014) Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res 55:803–811. https://doi.org/10.1093/jrr/rru017
Yuan H, Gaber MW, McColgan T, Naimark MD, Kiani MF, Merchant TE (2003) Radiation-induced permeability and leukocyte adhesion in the rat blood–brain barrier: modulation with anti-ICAM‑1 antibodies. Brain Res 969:59–69. https://doi.org/10.1016/S0006-8993(03)02278-9
Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR et al (2014) Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 53:1298–1304. https://doi.org/10.3109/0284186X.2014.957414
MacDonald SM, Laack NN, Terezakis S (2017) Humbling advances in technology: protons, brainstem necrosis, and the self-driving car. Int J Radiat Oncol Biol Phys 97:216–219. https://doi.org/10.1016/j.ijrobp.2016.08.001
Roberts KW, Wan Chan Tseung HS, Eckel LJ, Harmsen WS, Beltran C, Laack NN (2019) Biologic dose and imaging changes in pediatric brain tumor patients receiving spot scanning proton therapy. Int J Radiat Oncol Biol Phys 105:664–673. https://doi.org/10.1016/j.ijrobp.2019.06.2534
Gunther JR, Sato M, Chintagumpala M, Ketonen L, Jones JY, Allen PK et al (2015) Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 93:54–63. https://doi.org/10.1016/j.ijrobp.2015.05.018
Kralik SF, Ho CY, Finke W, Buchsbaum JC, Haskins CP, Shih C‑S (2015) Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am J Neuroradiol 36:1572–1578. https://doi.org/10.3174/ajnr.A4333
Niemierko A, Schuemann J, Niyazi M, Giantsoudi D, Maquilan G, Shih HA et al (2021) Brain necrosis in adult patients after proton therapy: Is there evidence for dependency on linear energy transfer? Int J Radiat Oncol Biol Phys 109:109–119. https://doi.org/10.1016/j.ijrobp.2020.08.058
Paganetti H (2017) Relating the proton relative biological effectiveness to tumor control and normal tissue complication probabilities assuming interpatient variability in α/β. Acta Oncol 56:1379–1386. https://doi.org/10.1080/0284186X.2017.1371325
Devine CA, Liu KX, Ioakeim-Ioannidou M, Susko M, Poussaint TY, Huisman TAGM et al (2019) Brainstem injury in pediatric patients receiving posterior fossa photon radiation. Int J Radiat Oncol Biol Phys 105:1034–1042. https://doi.org/10.1016/j.ijrobp.2019.08.039
Haas-Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T et al (2018) National Cancer Institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys 101:152–168. https://doi.org/10.1016/j.ijrobp.2018.01.013
Scharl S, Kirstein A, Kessel KA, Duma M‑N, Oechsner M, Straube C et al (2019) Cavity volume changes after surgery of a brain metastasis-consequences for stereotactic radiation therapy. Strahlenther Onkol 195:207–217. https://doi.org/10.1007/s00066-018-1387-y
Wald PM, Raval R, Guiou M (2016) Surgical cavity dynamics after resection of brain metastases and its implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys 96:E93–E94. https://doi.org/10.1016/j.ijrobp.2016.06.827
Jarvis LA, Simmons NE, Bellerive M, Erkmen K, Eskey CJ, Gladstone DJ et al (2012) Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys 84:943–948. https://doi.org/10.1016/j.ijrobp.2012.01.067
Kraus KM, Heath E, Oelfke U (2011) Dosimetric consequences of tumour motion due to respiration for a scanned proton beam. Phys Med Biol 56:6563–6581. https://doi.org/10.1088/0031-9155/56/20/003
Lambert J, Suchowerska N, McKenzie DR, Jackson M (2005) Intrafractional motion during proton beam scanning. Phys Med Biol 50:4853–4862. https://doi.org/10.1088/0031-9155/50/20/008
Szeto YZ, Witte MG, van Kranen SR, Sonke J‑J, Belderbos J, van Herk M (2016) Effects of anatomical changes on pencil beam scanning proton plans in locally advanced NSCLC patients. Radiother Oncol 120:286–292. https://doi.org/10.1016/j.radonc.2016.04.002
Müller BS, Duma MN, Kampfer S, Nill S, Oelfke U, Geinitz H et al (2015) Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Phys Med 31:266–272. https://doi.org/10.1016/j.ejmp.2015.02.007
El-Gamal FE-ZA, Elmogy M, Atwan A (2016) Current trends in medical image registration and fusion. Egypt Inform J 17:99–124. https://doi.org/10.1016/j.eij.2015.09.002
Eekers DB, In ’t Ven L, Roelofs E, Postma A, Alapetite C, Burnet NG et al (2018) The EPTN consensus-based atlas for CT- and MR-based contouring in neuro-oncology. Radiother Oncol 128:37–43. https://doi.org/10.1016/j.radonc.2017.12.013
Villemoes E (2018) Volumetric reconstruction and representation with applications in radiotherapy planning. Linkopings Universitet, Department of Physics, Chemistry and Biology
Zou KH, Warfield SK, Bharatha A, Tempany CMC, Kaus MR, Haker SJ et al (2004) Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 11:178–189
Fleiss J (1981) Statistical methods for rates and proportions, 2nd edn. Wiley, New York, pp 212–236
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J‑C, Pujol S et al (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Fjæra LF, Li Z, Ytre-Hauge KS, Muren LP, Indelicato DJ, Lassen-Ramshad Y et al (2017) Linear energy transfer distributions in the brainstem depending on tumour location in intensity-modulated proton therapy of paediatric cancer. Acta Oncol 56:763–768. https://doi.org/10.1080/0284186X.2017.1314007
Acharya S, Wang C, Quesada S, Gargone MA, Ates O, Uh J et al (2021) Adaptive proton therapy for pediatric patients: improving the quality of the delivered plan with on-treatment MRI. Int J Radiat Oncol Biol Phys 109:242–251. https://doi.org/10.1016/j.ijrobp.2020.08.036
Acknowledgements
Stefania Volpe MD was partially supported by the Italian Ministry of Health with Progetto di Eccellenza, and is a PhD student within the European School of Molecular Medicine (SEMM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
S. Volpe, P.-Y. Bondiau, L. Claude, A. Claren, L. Padovani, H. AlGhamdi, G. Duhil De Benaze, L. Opitz, G. Baudin, C. Dejean, D. Maneval, B.A. Jereczek-Fossa, and J. Doyen declare that they have no competing interests.
Additional information
J. Doyen is the author responsible for the statistical analysis.
Supplementary Information
Table S1:
Gross tumor volume extension—anatomical details
Rights and permissions
About this article
Cite this article
Volpe, S., Bondiau, PY., Claude, L. et al. Postsurgical geometrical variations of tumor bed and brainstem during photon and proton therapy for pediatric tumors of the posterior fossa: dosimetric impact and predictive factors. Strahlenther Onkol 197, 1113–1123 (2021). https://doi.org/10.1007/s00066-021-01828-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-021-01828-8