Abstract
Purpose
To investigate the performance of a knowledge-based RapidPlan, for optimisation of intensity-modulated proton therapy (IMPT) plans applied to hepatocellular cancer (HCC) patients.
Methods
A cohort of 65 patients was retrospectively selected: 50 were used to “train” the model, while the remaining 15 provided independent validation. The performance of the RapidPlan model was benchmarked against manual optimisation and was also compared to volumetric modulated arc therapy (RapidArc) photon plans. A subanalysis appraised the performance of the RapidPlan model applied to patients with lesions ≤300 cm3 or larger. Quantitative assessment was based on several metrics derived from the constraints of the NRG-GI003 clinical trial.
Results
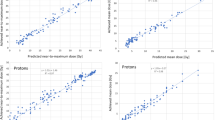
There was an equivalence between manual plans and RapidPlan-optimised IMPT plans, which outperformed the RapidArc plans. The planning dose–volume objectives were met on average for all structures except for D0.5 cm3 ≤30 Gy in the bowels. Limiting the results to the class-solution proton plans (all values in Gy), the data for manual plans vs RapidPlan-based IMPT plans, respectively, showed the following: D99% to the target of 47.5 ± 1.4 vs 47.2 ± 1.2; for organs at risk, the mean dose to the healthy liver was 6.7 ± 3.6 vs 6.7 ± 3.7; the mean dose to the kidneys was 0.2 ± 0.5 vs 0.1 ± 0.2; D0.5 cm3 for the bowels was 33.4 ± 16.4 vs 30.2 ± 16.0; for the stomach was 17.9 ± 19.9 vs 14.9 ± 18.8; for the oesophagus was 17.9 ± 15.1 vs 14.9 ± 13.9; for the spinal cord was 0.5 ± 1.6 vs 0.2 ± 0.7. The model performed similarly for cases with small or large lesions.
Conclusion
A knowledge-based RapidPlan model was trained and validated for IMPT. The results demonstrate that RapidPlan can be trained adequately for IMPT in HCC. The quality of the RapidPlan-based plans is at least equivalent compared to what is achievable with manual planning. RapidPlan also confirmed the potential to optimise the quality of the proton therapy results, thus reducing the impact of operator planning skills on patient results.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre L, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK (2002) Analysis of radiation induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 53:810–821
Wang PM, Hsu WC, Chung NN, Chang FL, Fogliata A, Cozzi L (2013) Radiotherapy with volumetric modulated arc therapy for hepatocellular carcinoma patients ineligible for surgery or ablative treatments. Strahlenther Onkol 189:301–307
Wang PM, Hsu WC, Chung NN, Chang FL, Fogliata A, Cozzi L (2012) Radiation treatment with volumetric modulated arc therapy of hepatocellular carcinoma patients. Early clinical outcome and toxicity profile from a retrospective analysis of 138 patients. Radiat Oncol 7:207
Yeung RH, Chapman TR, Bowen SR, Apisarnthanarax S (2017) Proton beam therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 17:911–924
Chuong MD, Kaiser A, Khan F, Parikh P, Ben-Josef E, Crane C et al (2019) Consensus report from the miami liver proton therapy conference. Front Oncol 9:457
Sanford NN, Pursley J, Noe B, Yeap BY, Goyal L, Clark JW et al (2019) Protons versus photons for unresectable hepatocellular carcinoma: liver decompensation and overall survival. Int J Radiat Oncol Biol Phys 105:64–72
Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS et al (2019) Does risk-adapted proton beam therapy have a role as a complementary or alternative therapeutic option for hepatocellular carcinoma? Cancers 11:2
Cozzi L, Comito T, Fogliata A, Franzese C, Tomatis S, Scorsetti M (2018) Critical appraisal of the potential role of intensity modulated proton therapy in the hypofractionated treatment of advanced hepatocellular carcinoma. PloS One 13:e0201992
Chanyavanich V, Das S, Lee W, Lo JY (2011) Knowledge-based IMRT treatment planning for prostate cancer. Med Phys 38:2515–2522
Zhu X, Ge Y, Li T, Thongphiew D, Yin FF, Wu Q (2011) A planning quality evaluation tool for prostate adaptive IMRT based on machine learning. Med Phys 38:719–726
Good D, Lo J, Lee R, Wu QJ, Yin FF, Das SK (2013) A knowledge-based approach to improving and homogenising intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys 87:176–181
Moore KL, Brame RS, Low DA, Mutic S (2011) Experience-based quality control of clinical intensity modulated radiotherapy planning. Int J Radiat Oncol Biol Phys 81:545–551
Appenzoller LM, Michalski JM, Thorstad WL, Mutic S, Moore KL (2012) Predicting dose-volume histograms for organs-at risk in IMRT planning. Med Phys 39:7446–7461
Ge Y, Wu QJ (2019) Knowledge-based planning for intensity-modulated radiation therapy: a review of data-driven approaches. Med Phys 46:2760–2775
Fogliata A, Wang PM, Belosi F, Clivio A, Nicolini G, Vanetti E et al (2014) Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol 9:236
Yu G, Li Y, Feng Z, Tao C, Yu Z, Li B et al (2018) Knowledge-based IMRT planning for individual liver cancer patients using a novel specific model. Radiat Oncol 13:52
Delaney AR, Dahele M, Tol JP, Kuijper IT, Slotman BJ, Verbakel W (2017) Using a knowledge-based planning solution to select patients for proton therapy. Radiother Oncol 124:263–270
Delaney AR, Verbakel WF, Lindberg J, Koponen TK, Slotman BJ, Dahele M (2018) Evaluation of an automated proton planning solution. Cureus 10:e3696
Vassiliev ON, Wareing TA, McGhee J, Failla G, Salehpour MR, Mourtada F (2010) Validation of a new grid-based Boltzmann equation solver for dose calculation in radiotherapy with photon beams. Phys Med Biol 55:581–598
Nocedal J, Wright SJ (2006) Numerical optimisation, 2nd edn.. ISBN 978-0-387-30303‑1
Langendijk JA, Boersma LJ, Rasch CRN, van Vulpen M, Reitsma JB, van der Schaaf A et al (2018) Clinical trial strategies to compare protons with photons. Semin Radiat Oncol 28:79–87
Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verhaij M (2013) Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 107:267–273
Rwigema JM, Langendijk JA, van der Laan HP, Lukens JN, Swisher-McClure SD, Lin A (2019) A model-based approach to predict short-term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 104:553–562
Blanchard P, Wong A, Gunn GB, Garden AS, Mohamed ASR, Rosenthal DI et al (2016) Toward a model-based patient selection strategy for proton therapy: external validation of photon-derived normal tissue complication probability models in a head and neck proton therapy cohort. Radiother Oncol 121:381–386
Cheng Q, Roelofs E, Ramaekers BL, Eekers D, van Soest J, Lustberg T et al (2016) Development and evaluation of an online three-level proton vs photon decision support prototype for head and neck cancer—comparison of dose, toxicity and cost-effectiveness. Radiother Oncol 118:281–285
Prayongrat A, Kobashi K, Ito YM, Katoh N, Tamura M, Dekura Y et al (2019) The normal tissue complication probability model-based approach considering uncertainties for the selective use of radiation modality in primary liver cancer patients. Radiother Oncol 135:100–106
International Commission on Radiation Units & Measurements (2010) Prescribing recording and reporting photon beam intensity modulated radiation therapy (IMRT) (ICRU Report 83)
Mizuhata M, Takamatsu S, Shibata S, Bou S, Sato Y, Kawamura M et al (2018) Respiratory-gated proton beam therapy for hepatocellular carcinoma adjacent to the gastrointestinal tract without fiducial markers. Cancers 10(2):58
Zhang Y, Huth I, Weber DC, Lomax AJ (2019) Dosimetric uncertainties as a result of temporal resolution in 4D dose calculations for PBS proton therapy. Phys Med Biol 64:125005
Zhang Y, Huth I, Weber DC, Lomax AJ (2018) A statistical comparison of motion mitigation performances and robustness of various pencil beam scanned proton systems for liver tumour treatments. Radiother Oncol 128:182–188
Bernatowicz K, Zhang Y, Perrin R, Weber DC, Lomax AJ (2017) Advanced treatment planning using direct 4D optimisation for pencil-beam scanned particle therapy. Phys Med Biol 62:6595–6609
Zhang Y, Huth I, Wegner M, Weber DC, Lomax AJ (2016) An evaluation of rescanning technique for liver tumour treatments using a commercial PBS proton therapy system. Radiother Oncol 121:281–287
Zhang Y, Knopf AC, Weber DC, Lomax AJ (2015) Improving 4D plan quality for PBS-based liver tumour treatments by combining online image guided beam gating with rescanning. Phys Med Biol 60:8141–8159
Bernatowicz K, Lomax AJ, Knopf A (2013) Comparative study of layered and volumetric rescanning for different scanning speeds of proton beam in liver patients. Phys Med Biol 58:7905–7920
Zhang Y, Boye D, Tanner C, Lomax AJ, Knopf A (2012) Respiratory liver motion estimation and its effect on scanned proton beam therapy. Phys Med Biol 57:1779–1795
Poulsen PR, Eley J, Langner U, Simone CB, Langen K (2018) Efficient interplay effect mitigation for proton pencil beam scanning by spot-adapted layered repainting evenly spread out over the full breathing cycle. Int J Radiat Oncol Biol Phys 100:226–234
Pfeiler T, Ahmad KD, Ayadi M, Bäumer C, Blanck O, Chan M et al (2018) Motion effects in proton treatments of hepatocellular carcinoma-4D robustly optimised pencil beam scanning plans versus double scattering plans. Phys Med Biol 63:235006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Cozzi, who is Clinical Research Scientist at Humanitas Cancer Center, acts as Scientific Advisor to Varian Medical Systems. R. Vanderstraeten is senior product manager for proton treatment planning at Varian Medical Systems. A. Fogliata, F.-L. Chang and P.-M. Wang declare that they have no competing interests.
Caption Electronic Supplementary Material
66_2020_1664_MOESM1_ESM.docx
Supplementary materials contains explanatory figure about the principal component analysys; additional figure about the model training; comparative figure for the average DVH for more OARs; the side analysis for small vs large lesions.
Rights and permissions
About this article
Cite this article
Cozzi, L., Vanderstraeten, R., Fogliata, A. et al. The role of a knowledge based dose–volume histogram predictive model in the optimisation of intensity-modulated proton plans for hepatocellular carcinoma patients. Strahlenther Onkol 197, 332–342 (2021). https://doi.org/10.1007/s00066-020-01664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01664-2