Abstract
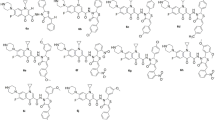
An efficient synthetic strategy was developed for the synthesis of hybrid pharmacophores; encompass the merging of β-lactams, 1,8-naphthyridine and secondary amines/pyridines. With a simple unified synthetic protocol, we prepared a series of substituted 3-chloro-1-((3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)amino)azetidin-2-one compounds (8a–8j) and evaluated for their antimicrobial, and anticancer activities (against MDA-MB-231 cell line). Notably, compounds 8d, 8g, and 8j were identified as potent anticancer molecules and comparable to Cisplatin. Compounds 8d and 8g were found to have promising antitubercular efficacy even greater than the standards Streptomycin and Ciprofloxacin. Compounds 8a, 8d, 8g, 8i, and 8j also displayed potency against both Gram-positive and Gram-negative bacteria and comparable to Ampicillin & Ciprofloxacin. Further, the biological activities were supported and studied by DFT studies and ADMET predictions.
Graphical Abstract





Similar content being viewed by others
References
Lazar C, Kluczyk A, Kiyota T, Konishi Y. Drug Evolution Concept in Drug Design: 1. Hybridization Method. J Med Chem. 2004;47:6973–82.
Ameri A, Khodarahmi G, Forootanfar H, Hassanzadeh F, Hakimelahi GH. Hybrid Pharmacophore Design, Molecular Docking, Synthesis, and Biological Evaluation of Novel Aldimine-Type Schiff Base Derivatives as Tubulin Polymerization Inhibitor. Chem Biodivers. 2018;15:e1700518.
Singh RK, Prasad DN, Bhardwaj TR. Hybrid pharmacophore-based drug design, synthesis, and antiproliferative activity of 1,4-dihydropyridines-linked alkylating anticancer agents. Med Chem Res. 2015;24:1534–45.
Kerru N, Gummidi L, Maddila S, Gangu KK, Jonnalagadda SB. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules. 2020;25:1909.
Heravi MM, Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020;10:44247–311.
Parmar DR, Soni JY, Guduru R, Rayani RH, Kusurkar RV, Vala AG, et al. Discovery of new anticancer thiourea-azetidine hybrids: design, synthesis, in vitro antiproliferative, SAR, in silico molecular docking against VEGFR-2, ADMET, toxicity, and DFT studies. Bioorg Chem. 2021;115:105206.
Parmar DR, Rayani RH, Vala AG, Kusurkar RV, Manvar RK, Talukdar SN, et al. Design, Synthesis, In Silico Studies and In Vitro Anticancer Activity of 3-(4-Methoxyphenyl)azetidine Derivatives. ChemistrySelect. 2020;5:14296–302.
Tahlan K, Jensen SE. Origins of the β-lactam rings in natural products. J Antibiot. 2013;66:401–10.
Kong KF, Schneper L, Mathee K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS. 2010;118:1–36.
Mehta PD, Sengar NPS, Pathak AK. 2-Azetidinone - A new profile of various pharmacological activities. Eur J Med Chem. 2010;45:5541–60.
Brandi A, Cicchi S, Cordero FM. Novel Syntheses of Azetidines and Azetidinones. Chem Rev. 2008;108:3988–4035.
Sharma R, Samadhiya P, Srivastava SD, Srivastava SK. Synthesis and pharmaceutical importance of 2-azetidinone derivatives of phenothiazine. J Chem Sci. 2012;124:633–7.
Slusarchyk WA, Bolton SA, Hartl KS, Huang MH, Jacobs G, Meng W, et al. Synthesis of potent and highly selective inhibitors of human tryptase. Bioorg Med Chem Lett. 2002;12:3235–8.
Burnett DA, Caplen MA, Davis HR, Burrier RE, Clader JW. 2-Azetidinones as Inhibitors of Cholesterol Absorption. J Med Chem. 1994;37:1733–6.
Kayarmar R, Nagaraja GK, Naik P, Manjunatha H, Revanasiddappa BC, Arulmoli T. Synthesis and characterization of novel imidazoquinoline based 2-azetidinones as potent antimicrobial and anticancer agents. J Saudi Chem Soc. 2017;21:S434–S444.
Patel NB, Pathak KK. Pyridoquinolones containing azetidinones: synthesis and their biological evaluation. Med Chem Res. 2012;21:2044–55.
Gervois P, Fruchart JC, Staels B. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Clin Pract Endocrinol Metab. 2007;3:145–56.
Kumar A, Rajput CS, Bhati SK. Synthesis of 3-[4′-(p-chlorophenyl)-thiazol-2′-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg Med Chem. 2007;15:3089–96.
Bhati SK, Kumar A. Synthesis of new substituted azetidinoyl and thiazolidinoyl-1,3,4-thiadiazino (6,5-b)indoles as promising anti-inflammatory agents. Eur J Med Chem. 2008;43:2323–30.
Tozser J, Oroszlan S. Proteolytic events of HIV-1 replication as targets for therapeutic intervention. Curr Pharm Des. 2003;9:1803.
Sakram B, Ravi D, Raghupathi M, Kumar BS, Anantha Lakshmi PV. A facile greener synthesis, antimicrobial evaluation and molecular modelling of new 4-aryl-2-(3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)phthalazin-1(2H)-one derivatives. Res Chem Intermed. 2019;45:2007–22.
Madaan A, Verma R, Kumar V, Singh AT, Jain SK, Jaggi M. 1,8-Naphthyridine Derivatives: A Review of Multiple Biological Activities. Arch Pharm Chem Life Sci. 2015;348:837–60.
Singh IP, Kumar S, Gupta S. Naphthyridines with Antiviral Activity - A Review. Med Chem. 2017;13:430–8.
Kiselev E, Dexheimer TS, Pommier Y, Cushman M. synthesis, and evaluation of dibenzo[c,h][1,6]naphthyridines as topoisomerase I inhibitors and potential anticancer agents. J Med Chem. 2010;53:8716–26.
Roseman KA, Gould MM, Linfield MW, Edwards BE. Antimalarials. 8-chloro-4-(2’-N,N-dibutylamino-1’-hydroxyethyl) benzo[h]-1,6-naphthyridine. J Med Chem. 1970;13:230–3.
Austin NE, Hadley MS, Harling JD, Harrington FP, Macdonald GJ, Mitchell DJ, et al. The design of 8,8-dimethyl[1,6]naphthyridines as potential anticonvulsant agents. Bioorg Med Chem Lett. 2003;13:1627–9.
Ravichandran V, Shalini S, Sundram K, Sokkalingam AD. QSAR study of substituted 1,3,4-oxadiazole naphthyridines as HIV-1 integrase inhibitors. Eur J Med Chem. 2010;45:2791–7.
Fu L, Feng X, Wang JJ, Xun Z, Hu JD, Zhang JJ, et al. Efficient Synthesis and Evaluation of Antitumor Activities of Novel Functionalized 1,8-Naphthyridine Derivatives. ACS Combin Sci. 2015;17:24–31.
Al-Romaizan AN, Jaber TS, Ahmed NS. Novel 1,8-Naphthyridine Derivatives: Design, Synthesis and in vitro screening of their cytotoxic activity against MCF7 cell line. Open Chem. 2019;17:943–54.
Grossi G, Di Braccio M, Roma G, Ballabeni V, Tognolini M, Barocelli E. 1,8-Naphthyridines V. Novel N-substituted 5-amino-N,N-diethyl-9-isopropyl [1,2,4]triazolo[4,3-a] [1,8]naphthyridine-6-carboxamides, as potent anti-inflammatory and/or analgesic agents completely devoid of acute gastrolesivity. Eur J Med Chem. 2005;40:155–65.
Surivet JP, Lange R, Hubschwerlen C, Keck W, Specklin JL, Ritz D, et al. Structure-guided design, synthesis and biological evaluation of novel DNA ligase inhibitors with in vitro and in vivo anti-staphylococcal activity. Bioorg Med Chem Lett. 2012;22:6705–11.
Gurjar VK, Pal D. Design, in silico studies, and synthesis of new 1,8-naphthyridine-3-carboxylic acid analogues and evaluation of their H1R antagonism effects. RSC Adv. 2020;10:13907–21.
Ferrarini PL, Mori C, Badawneh M, Calderone V, Calzolari L, Loffredo T, et al. Synthesis of 1,8-naphthyridine derivatives: Potential antihypertensive agents - Part VII. Eur J Med Chem. 1998;33:383–97.
Olepu S, Suryadevara PK, Rivas K, Yokoyama K, Verlinde CL, Chakrabarti D, et al. 2-Oxo-tetrahydro-1,8-naphthyridines as selective inhibitors of malarial protein farnesyltransferase and as anti-malarials. Bioorg Med Chem Lett. 2008;18:494–7.
Ferrarini PL, Mori C, Badawneh M, Franconi F, Manera C, Miceli M, et al. Synthesis and antiplatelet activity of some 3-phenyl-1,8-naphthyridine derivatives. Il Farmaco. 2000;55:603–10.
Santilli AA, Scotese AC, Bauer RF, Bell SC. 2-Oxo-1,8-naphthyridine-3-carboxylic acid derivatives with potent gastric antisecretory properties. J Med Chem. 1987;30:2270–7.
Abbas JA, Stuart RK. Vosaroxin: a novel antineoplastic quinolone. Expert Opin Investig Drugs. 2012;21:1223–33.
Fernández-Mato A, Blanco G, Quintela JM, Peinador C. Synthesis of new bis(2-[1,8]naphthyridinyl) bridging ligands with multidentate binding sites. Tetrahedron. 2008;64:3446–56.
Che C-M, Wan C-W, Ho K-Y, Zhou Z-Y. Strongly luminescent metal-organic compounds: spectroscopic properties and crystal structure of substituted 1,8-naphthyridine and its zinc(II) complex. New J Chem. 2001;25:63–5.
Fu WF, Jia LF, Mu WH, Gan X, Zhang JB, Liu PH, et al. Synthesis, characterization, photoinduced isomerization, and spectroscopic properties of vinyl-1,8-naphthyridine derivatives and their copper(I) complexes. Inorg Chem. 2010;49:4524–33.
Yu M-M, Li Z-X, Wei L-H, Wei D-H, Tang M-S. A 1,8-Naphthyridine-Based Fluorescent Chemodosimeter for the Rapid Detection of Zn2+ and Cu2+. Org Lett. 2008;10:5115–8.
Zhou Y, Xiao Y, Qian X. A highly selective Cd2+ sensor of naphthyridine: fluorescent enhancement and red-shift by the synergistic action of forming binuclear complex. Tetrahedron Lett. 2008;49:3380–4.
Vitaku E, Smith DT, Njardarson JT. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J Med Chem. 2014;57:10257–74.
Pandya KM, Battula S, Naik PJ. Pd-catalyzed post-Ugi intramolecular cyclization to the synthesis of isoquinolone-pyrazole hybrid pharmacophores & discover their antimicrobial and DFT studies. Tetrahedron Lett. 2021;81:153353.
Pandya KM, Battula S, Patel NB. Design & Synthesis of InCl3 catalyzed novel pyrazole conjugates with 2°-amines; discover their in-vitro antimicrobial & DFT studies. Polycycl Aromat Compd. 2021;43:1232–46.
Deep A, Kumar P, Narasimhan B, Lim SM, Ramasamy K, Mishra RK, et al. 2-Azetidinone Derivatives: Synthesis, Antimicrobial, Anticancer Evaluation and Qsar Studies. Acta Pol Pharm. 2016;73:65–78.
Sankar PS, Divya K, Padmaja A, Padmavathi V. Synthesis and Antimicrobial Activity of Azetidinone and Thiazolidinone Derivatives from Azolylindolyl Schiff’s Bases. Med Chem. 2017;7:340–7.
Lewin CS. Antibacterial activity of a 1,8-naphthyridine quinolone, PD 131628. J Med Microbiol. 1992;36:353–7.
Leonard JT, Gangadhar R, Gnanasam SK, Ramachandran S, Saravanan M, Sridhar SK. Synthesis and Pharmacological Activities of 1,8-Naphthyridine Derivatives. Biol Pharm Bull. 2002;25:798–802.
Barker D, Lee S, Varnava KG, Sparrow K, van Rensburg M, Deed RC, et al. Synthesis and Antibacterial Analysis of Analogues of the Marine Alkaloid Pseudoceratidine. Molecules. 2020;25:2713.
Dinarvand M, Spain M. Identification of Bioactive Compounds from Marine Natural Products and Exploration of Structure-Activity Relationships (SAR). Antibiotics. 2021;10:337.
Serafim MSM, Lavorato SnN, Kronenberger T, Sousa YV, Oliveira GP, dos Santos SG, et al. Antibacterial activity of synthetic 1,3-bis(aryloxy)propan-2-amines against Gram-positive bacteria. MicrobiologyOpen. 2019;8:e814.
Shastri LA, Shastri SL, Bathula CD, Basanagouda M, Kulkarni MV. Mild, Simple, and Efficient Method for N-Formylation of Secondary Amines via Reimer–Tiemann Reaction. Synth Commun. 2011;41:476–84.
Upadhyaya RS, Vandavasi JK, Rao VN, Vivek S, Dixit SS, Chattopadhyaya J. Design, synthesis, biological evaluation and molecular modelling studies of novel quinoline derivatives against Mycobacterium tuberculosis. Bioorg Med Chem. 2009;17:2830–41.
Hursthouse MB, Hughes DS, Gelbrich T, Threlfall TL. Describing hydrogen-bonded structures; topology graphs, nodal symbols and connectivity tables, exemplified by five polymorphs of each of sulfathiazole and sulfapyridine. Chem Cent J. 2015;9:1.
Miar M, Shiroudi A, Pourshamsian K, Oliaey AR, Hatamjafari F. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo[d]thiazole-2(3H)-imine and its para-substituted derivatives: Solvent and substituent effects. J Chem Res. 2020;45:147–58.
Tanwar OP, Saha R, Marella A, Alam M, Akhter M, Dua VK. Prediction and comparison of drug likeliness properties of primaquine and its structural analogues using In-Silico ADME and Toxicity Prediction Tools. J Adv Bioinform Appl Res. 2014;5:172–82.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;46:3–26.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64:4–17.
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem. 2002;45:2615–23.
Acknowledgements
KMP thanks Dr Benjamin R. Boswell, Department of Chemistry, Stanford University, Stanford, CA, USA for his support in the final corrections of paper. NBP thanks UGC, New Delhi, for the UGC/BSR faculty fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandya, K.M., Battula, S., Kumar, K.A.A. et al. Design & synthesis of hybrid pharmacophore of β-lactam, 1,8-naphthyridine, and secondary amines; Discover their in vitro antimicrobial, anticancer properties & DFT and ADMET prediction studies. Med Chem Res 32, 1098–1108 (2023). https://doi.org/10.1007/s00044-023-03058-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03058-2