Abstract
Alzheimer’s disease (AD), a worldwide mental disorder manifested with dementia symptoms, was used to be treated with tacrine. The latter use was terminated recently due to its hepatotoxicity. Thus, synthesis of tacrine analogues free of hepatotoxicity was attempted, a group of new 2-oxo-1,2-dihydroquinoline-3-carboxamides have been synthesized and properly characterized. Their biological evaluation in inhibiting acetylcholinesterase enzyme (AChE), was evaluated by the in-house gas chromatography method. The synthesized carboxamides showed a strong potency in inhibiting AChE. Nine compounds from the group had activities higher than or close to donepezil (the positive control, IC50 ca 10 nM). For example, compound 7 depicted IC50 of 7 nM and was subsequently evaluated by the in vivo zebra fish model. Interestingly compound 7 showed better recovery from scopolamine-induced dementia than that of donepezil. Further ex vivo and in vivo models have been established recently in the lab to evaluate the safety of these compounds.

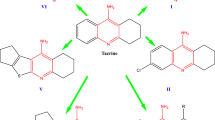
Graphical abstract






Similar content being viewed by others
References
Liu L, Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res Rev. 2014;15:6–15.
Boccardi V, Murasecco I, Mecocci P. Diabetes Drugs in The Fight Against Alzheimer's Disease. Ageing Res Rev. 2019;54:1–35.
Giaume C, Sáez JC, Song W, Leybaert L, Naus CC. Connexins and pannexins in Alzheimer’s disease. Neurosci Lett. 2019;695:100–5.
Das N, Raymick J, Sarkar S. Role of metals in Alzheimer’s disease. Metab Brain Dis. 2021;36:1627–39.
Al-Shaikh FSH, Duara R, Crook JE, Lesser ER, Schaeverbeke J, Hinkle KM, et al. Selective vulnerability of the nucleus basalis of Meynert among neuropathologic subtypes of Alzheimer disease. JAMA Neurol. 2020;77:225–33.
Chen Y, Shu K, Kang H. Deep Brain Stimulation in Alzheimer’s Disease: Targeting the Nucleus Basalis of Meynert. J Alzheimer’s Dis. 2021;80:53–70.
Mahady L, Nadeem M, Malek‐Ahmadi M, Chen K, Perez SE, Mufson EJ. HDAC 2 dysregulation in the nucleus basalis of Meynert during the progression of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2019;45:380–97.
Alexandris AS, Walker L, Liu AKL, McAleese KE, Johnson M, Pearce RKB, et al. Cholinergic deficits and galaninergic hyperinnervation of the nucleus basalis of Meynert in Alzheimer’s disease and Lewy body disorders. Neuropathol Appl Neurobiol. 2020;46:264–78.
Dani M, Wood M, Mizoguchi R, Fan Z, Walker Z, Morgan R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain. 2018;141:2740–54.
Humpel C. Platelets: their potential contribution to the generation of beta-amyloid plaques in Alzheimer’s disease. Curr Neurovasc Res. 2017;14:290–8.
Cai H-Y, Yang J-T, Wang Z-J, Zhang J, Yang W, Wu M-N, Qi J-S. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2018;495:1034–40.
Muir JL. Acetylcholine, aging, and Alzheimer’s disease. Pharmacol Biochem Behav. 1997;56:687–96.
Tropea MR, Puma DDL, Melone M, Gulisano W, Arancio O, Grassi C, et al. Genetic deletion of α7 nicotinic acetylcholine receptors induces an age-dependent Alzheimer’s disease-like pathology. Prog Neurobiol. 2021;206:102154.
Komersová A, Kovářová M, Komers K, Lochař V, Čegan A. Why is the hydrolytic activity of acetylcholinesterase pH dependent? Kinetic study of acetylcholine and acetylthiocholine hydrolysis catalyzed by acetylcholinesterase from electric eel. Z für Naturforsch C. 2018;73:345–51.
Kaur A, Anand C, Singh TG, Dhiman S, Babbar R. Acetylcholinesterase inhibitors: a milestone to treat neurological disorders. Plant Arch. 2019;19:1347–59.
H Ferreira-Vieira T, M Guimaraes I, R Silva F, M Ribeiro F. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 2016;14:101–15.
Abubakar MU, Abubakar D. Characterization of Acetylcholinesterase from Various Sources: A Mini Re. J Environ Bioremediation Toxicol. 2021;4:24–30.
Atack JR, Perry EK, Bonham JR, Candy JM, Perry RH. Molecular forms of acetylcholinesterase and butyrylcholinesterase in the aged human central nervous system. J Neurochem. 1986;47:263–77.
Moss DE. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Are irreversible inhibitors the future? Int J Mol Sci. 2020;21:3438.
Haake A, Nguyen K, Friedman L, Chakkamparambil B, Grossberg GT. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin Drug Saf. 2020;19:147–57.
Marucci G, Buccioni M, Dal Ben D, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021;190:108352.
Li K, Jiang Y, Li G, Liu T, Yang Z. Novel Multitarget Directed Tacrine Hybrids as Anti-Alzheimer’s Compounds Improved Synaptic Plasticity and Cognitive Impairment in APP/PS1 Transgenic Mice. ACS Chem Neurosci. 2020;11:4316–28.
Nikseresht A, Ghasemi S, Parak S. [Cu3 (BTC) 2]: A metal–organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedländer reaction under conventional and ultrasound irradiation. Polyhedron 2018;151:112–7.
Makhaeva GF, Kovaleva NV, Boltneva NP, Lushchekina SV, Astakhova TY, Rudakova EV, et al. New hybrids of 4-amino-2, 3-polymethylene-quinoline and p-tolylsulfonamide as dual inhibitors of acetyl-and butyrylcholinesterase and potential multifunctional agents for Alzheimer’s disease treatment. Molecules. 2020;25:3915.
McEneny-King A, Osman W, Edginton AN, Rao PPN. Cytochrome P450 binding studies of novel tacrine derivatives: predicting the risk of hepatotoxicity. Bioorg Med Chem Lett. 2017;27:2443–9.
Yip LY, Aw CC, Lee SH, Hong YS, Ku HC, Xu WH, et al. The liver-gut microbiota axis modulates hepatotoxicity of tacrine in the rat. Hepatology 2018;67:282–95.
Li Z, Mei J, Jiang L, Geng C, Li Q, Yao X, Cao J. Chaga medicinal mushroom, Inonotus obliquus (Agaricomycetes) polysaccharides suppress tacrine-induced apoptosis by ROS-scavenging and mitochondrial pathway in HepG2 cells. Int J Med Mushrooms. 2019;21:583–93.
Siraki AG. Free radical metabolites in arylamine toxicity. Adv Mol Toxicol. 2013;7:39–82.
Sim E, Laurieri N. Arylamine N-acetyltransferases in Health And Disease: From Pharmacogenetics To Drug Discovery and Diagnostics. World Scientific. 3–42 (2018).
Liu X, Liu Y, Zhao G, Zhang Y, Liu L, Wang J, et al. Biochemical Characterization of Arylamine N-acetyltransferases From Vibrio vulnificus. Front Microbiol. 2021;11:3405
Blömeke B, Lichter J. Expression and Activity of Arylamine N-Acetyltransferases in Organs: Implications on Aromatic Amine Toxicity. In: ARYLAMINE N-ACETYLTRANSFERASES IN HEALTH AND DISEASE: From Pharmacogenetics to Drug Discovery and Diagnostics, World Scientific, 133–64 (2018).
Agúndez JAG, García-Martín E Human Arylamine N-Acetyltransferase Type 2: Phenotypic Correlation with Genotype-A Clinical Perspective. In: Arylamine N-acetyltransferases in health and disease: from pharmacogenetics to drug discovery and diagnostics, World Scientific, 69–89 (2018).
Fu J, Bao F, Gu M, Liu J, Zhang Z, Ding J, et al. Design, synthesis and evaluation of quinolinone derivatives containing dithiocarbamate moiety as multifunctional AChE inhibitors for the treatment of Alzheimer’s disease. J Enzym Inhib Med Chem. 2020;35:118–28.
Pavlidis N, Kofinas A, Papanikolaou MG, Miras HN, Drouza C, Kalampounias AG, et al. Synthesis, characterization and pharmacological evaluation of quinoline derivatives and their complexes with copper (ΙΙ) in in vitro cell models of Alzheimer’s disease. J Inorg Biochem. 2021;217:111393.
Hepnarova V, Korabecny J, Matouskova L, Jost P, Muckova L, Hrabinova M, et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer's disease. Eur J Med Chem. 2018;150:292–306.
de Oliveira C Brum J, Neto DCF, de Almeida JSFD, Lima JA, Kuca K, França TCC, et al. Synthesis of new quinoline-piperonal hybrids as potential drugs against Alzheimer’s disease. Int J Mol Sci. 2019;20:3944.
Worek F, Eyer P, Thiermann H. Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal 2012;4:282–91.
Holas O, Musilek K, Pohanka M, Kuca K. The progress in the cholinesterase quantification methods. Expert Opin Drug Disco. 2012;7:1207–23.
Komers K, Komersová A, Stratilová J. Comment on colorimetric monitoring of enzymatic hydrolysis of acetylthiocholine. Sci. Pap. Univ. Pardubice. Ser. A, Fac. Chem. Technol. 2003;9:89–96.
Gerlai R. Editorial: A small fish with a big future: Zebrafish in behavioral neuroscience: Rev. Neurosci. Rev Neurosci. 2011;22:3–4.
Newman M, Ebrahimie E, Lardelli M. Using the zebrafish model for Alzheimer’s disease research. Front Genet. 2014;5:1–10.
Alzweiri M, Al-Helo T. Gas Chromatography with Modified pH-Sensitive Pellets in Evaluating Esterase Activity of Carbonic Anhydrase III Enzyme: drug discovery approach. Chromatographia. 2021;84:1–8.
Sabbah DA, Hishmah B, Sweidan K, Bardaweel S, AlDamen M, Zhong HA, et al. Structure-based design: Synthesis, X-ray crystallography, and biological evaluation of N-substituted-4-hydroxy-2-quinolone-3-carboxamides as potential cytotoxic agents. Anti-Cancer Agents Med Chem (Former Curr Med Chem Agents). 2018;18:263–76.
Bollo S, Munoz L, Núñez‐Vergara LJ, Squella JA. Electrochemical characterization of tacrine, an antialzheimer’s disease drug, and its determination in pharmaceuticals. Electroanal Int J Devoted Fundam Pract Asp Electroanal 2000;12:376–82.
Feixas F, Matito E, Sola M, Poater J. Analysis of Hückel’s [4 n+ 2] Rule through Electronic Delocalization Measures. J Phys Chem A 2008;112:13231–8.
Ogura H, Kosasa T, Kuriya Y, Yamanishi Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmacol 2000;22:609–14.
Recanatini M, Cavalli A, Belluti F, Piazzi L, Rampa A, Bisi A, et al. SAR of 9-amino-1, 2, 3, 4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity, QSAR, and structure-based CoMFA of tacrine analogues. J Med Chem. 2000;43:2007–18.
Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5, 6-dimethoxy-1-oxoindan-2-yl) methyl] piperidine hydrochloride and related compounds. J Med Chem.1995;38:4821–9.
Ota T, Shinotoh H, Fukushi K, Kikuchi T, Sato K, Tanaka N, et al. Estimation of plasma IC50 of donepezil for cerebral acetylcholinesterase inhibition in patients with Alzheimer disease using positron emission tomography. Clin Neuropharmacol. 2010;33:74–78.
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
Hamilton TJ, Morrill A, Lucas K, Gallup J, Harris M, Healey M, et al. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci Rep. 2017;7:1–9.
Acknowledgements
We wish to thank The University of Jordan represented by the Deanship of Academic Research for supporting and funding the project of (62/2019-2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Alzweiri, M., Sweidan, K., Saleh, O.a. et al. Synthesis and evaluation of new 2-oxo-1,2-dihydroquinoline-3-carboxamides as potent inhibitors against acetylcholinesterase enzyme. Med Chem Res 31, 1448–1460 (2022). https://doi.org/10.1007/s00044-022-02922-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02922-x