Abstract
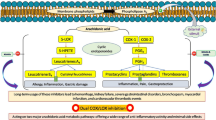
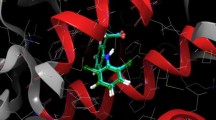
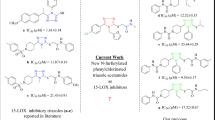
Series of the 3-methylquinazoline 3-oxide derivatives were evaluated through enzymatic assays in vitro and in silico for potential inhibitory effect against cyclooxygenase-1/2 (COX-1/2) and lipoxygenase-5 activities as well as for free radical scavenging potential and cytotoxicity. The 6-bromo (3k) and 6-iodo substituted 2-(4-chlorophenyl)-4-methylquinazoline 3-oxide (3q) exhibited significant inhibitory effect against both COX-1 (IC50 = 13.9 ± 3.21 µM and 9.7 ± 0.09 µM, respectively) and COX-2 (IC50 = 6.4 ± 0.74 µM and 4.6 ± 1.45 µM, respectively) compared to quercetin (IC50 = 13.84 ± 1.57 µM and 5.06 ± 2.60 µM, respectively). Their activity was, however, modest compared to the selective COX-2 inhibitor, celecoxib, with IC50 values of 7.35 ± 0.88 µM and 0.62 ± 0.74 µM against COX-1 and COX-2, respectively. The two compounds exhibited comparable effect on LOX-5 (IC50 = 15.0 µM), which is moderate compared to quercetin (IC50 = 8.04 ± 1.12 µM) and modest against zileuton (IC50 = 0.42 ± 0.51 µM). Structure–activity relationship analysis suggested that the presence of a halogen atom at the C-6 position and a 2-aryl group enhance inhibitory effect against COX-2. This observation is well supported by molecular docking studies.








Similar content being viewed by others
Data availability
The CIF files containing complete information on the studied structures were deposited with the Cambridge Crystallographic Data Center, 3b (CCDC 2035791) and 3c (CCDC 2035790), and are freely available upon request from the following website: www.ccdc.cam.ac.uk/datarequest/cif or by contacting the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033; email: deposit@ccdc.cam.ac.uk.
References
Kishore N, Kumar P, Shanker K, Verma AK. Human disorders associated with inflammation and the evolving role of natural products to overcome. Eur J Med Chem. 2019;179:272–309. https://doi.org/10.1016/j.ejmech.2019.06.034.
Rani V, Deep G, Singh RK, Palle K, Yadav UCS. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;1148:183–93. https://doi.org/10.1016/j.lfs.2016.02.002.
Brubaker L, Kendall L, Reina E. Multimodal analgesia: a systematic review of local NSAIDs for non-ophthalmologic post operative pain management. Int J Surg. 2016;32:158–66. https://doi.org/10.1016/j.ijsu.2016.07.003.
Qandeel NA, El-Damasy AK, Sharawy MH, Bayomi SM, El-Gohary NS. Synthesis, in vivo anti-inflammatory, COX-1/COX-2 and 5-LOX inhibitory activities of new 2,3,4-trisubstituted thiophenes. Bioorg Chem. 2020;101:103890 https://doi.org/10.1016/j.bioorg.2020.103890R.
Liaras K, Fesatidou M, Geronikaki A. Thiazoles and thiazolidinones as COX/LOX inhibitors. Molecules. 2018;23:685 https://doi.org/10.3390/molecules23030685.
Ju Z, Su M, Hong J, Kim EL, Moon HR, Chung HY, et al. Design of balanced COX inhibitors based on anti-inflammatory and/or COX-2 inhibitory ascidian metabolites. Eur J Med Chem. 2019;180:86–98. https://doi.org/10.1016/j.ejmech.2019.07.016.
Rawat C, Kukal S, Dahiya UR, Kukreti R. Cyclooxygenase-2 (COX-2) inhibitors: future therapeutic strategies for epilepsy management. J Neuroinflamm. 2019;16197. https://doi.org/10.1186/s12974-019-1592-3.
Rathee P, Chaudhary H, Rathee S, Kumar V, Kohli K. Mechanism of action flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229–35. https://doi.org/10.2174/187152809788681029.
Alagarsamy V, Chitra K, Saravanan G, Solomon VR, Sulthana MT, Narendhar B. An overview of quinazolines: pharmacological significance and recent developments. Eur J Med Chem. 2018;151:628–85. https://doi.org/10.1016/j.ejmech.2018.03.076.
Pathare RS, Maurya AK, Kumari A, Agnihotri VK, Vermac VP, Sawant DM. Synthesis of quinazoline-3-oxides via a Pd(II) catalyzed azide–isocyanide coupling/cyclocondensation reaction. Org Biomol Chem. 2019;17:363–8. https://doi.org/10.1039/C8OB02627K.
Combs DW, Rampulla MS, Russell RK, Rampulla R, Klaubert DH, Ritchie D, et al. Design, synthesis and bronchodilatory activity of a series of quinazoline-3-oxides. Drug Des Deliv. 1990;6:241–54.
Alzogaray RA, Fontán A, Camps F, Masuh H, Orihuela PS, Fernández D, et al. Behavioural response of Triatoma infestans (Klug) (Hemiptera: Reduviidae) to quinazolines. Molecules. 2005;10:1190–6. https://doi.org/10.3390/molecules1009119.
Armarego WLF. Quinazolines. Part V. Covalent hydration in quinazoline 3-oxides. J Chem Soc. 1962:5030–6. https://doi.org/10.1039/JR9620005030.
Heaney F, Lawless E. 2-Vinyl quinazoline 3-oxide; preparation from acid induced cyclocondensation of 2-acylaminoaryloximes. J Heterocycl Chem 2007;44:569–74. https://doi.org/10.1002/jhet.5570440311.
Jatangi N, Palakodety RK. I2-Catalyzed oxidative synthesis of N,4-disubstituted quinazolines and quinazoline oxides. Org Biomol Chem. 2019;17:3714–7. https://doi.org/10.1039/C9OB00349E.
Kovendi A, Kircz M. New synthesis for quinazoline N3-oxides and 1,2-dihydroquinazoline N3-oxides. Chem Ber. 1965;98:1049–59.
Madabhushi S, Mallu KKR, Jillella R, Kurva S, Singh R. One-step method for synthesis of 2,4-disubstituted quinazoline 3-oxides by reaction of a 2-aminoaryl ketone with a hydroxamic acid using Zn(OTf)2 as the catalyst. Tetrahedron Lett. 2014;55:1979–82. https://doi.org/10.1016/j.tetlet.2014.01.150.
Chen Y-C, Yang D-Y. Visible light-mediated synthesis of quinazolines from 1,2-dihydro- quinazoline-3-oxides. Tetrahedron. 2013;69:10438–44. https://doi.org/10.1016/j.tet.2013.09.089.
Eynde JJV, Godin J, Mayence A, Maquestiau A, Anders E. A new and convenient method for the preparation of 2-substituted quinazolines. Synthesis. 1993;9:867–9. https://doi.org/10.1055/S-1993-25958.
Coșkun N, Cetin M. A new regioselective synthesis and ambient light photochemistry of quinazoline 1-oxides. Tetrahedron. 2007;63:2966–72. https://doi.org/10.1016/j.tet.2007.02.004.
Samandram R, Korukçu MÇ, Coşkun N. Eco-friendly H2O2 oxidation of 1,2- dihydroquinazoline-3-oxides to quinazoline-3-oxides. Synth Commun. 2021;51:2349–56. https://doi.org/10.1080/00397911.2021.1934876.
Ye X, Chen Z, Zhang Z, Fu Y, Deng Z, Peng Y. A convenient approach to 2,4-disubstituted quinazoline-3-oxides using active MnO2 as the oxidant. Can J Chem. 2019;97:682–9. https://doi.org/10.1139/cjc-2018-0521.
Wang Q, Wang F, Yang X, Zhou X, Li X. Rh(III)- and Zn(II)-catalyzed synthesis of quinazoline N‑oxides via C−H amidation−cyclization of oximes. Org Lett. 2016;18:6144–7. https://doi.org/10.1021/acs.orglett.6b03155.
Biswass SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:20165698931. https://doi.org/10.1155/2016/5698931.
Mphahlele MJ, Makhafola TJ, Mmonwa MM. In vitro cytotoxicity of novel 2,5,7-tricarbo-substituted indoles derived from 2-amino-5-bromo-3-iodoacetophenone. Bioorg Med Chem. 2016;24:4576–86. https://doi.org/10.1016/j.bmc.2016.07.056.
Counceller CM, Eichman CC, Wray BC, Stambuli JP. A practical metal-free synthesis of 1H-indazoles. Org Lett. 2008;10:1021–3. https://doi.org/10.1021/ol800053f.
Mphahlele MJ, Magwaza NM, Gildenhuys S, Setshedi IB. Synthesis, α-glucosidase inhibition and antioxidant activity of the 7-carbo–substituted 5-bromo-3-methylindazoles. Bioorg Chem. 2020;97:103702 https://doi.org/10.1016/j.bioorg.2020.10370.
Wray BC, Stambuli JP. Synthesis of N-arylindazoles and benzimidazoles from a common intermediate. Org Lett. 2010;12:4576–9. https://doi.org/10.1021/ol101899q.
López SE, Salazar J. Trifluoroacetic acid: Uses and recent applications in organic synthesis. J Fluor Chem. 2013;156:73–100. https://doi.org/10.1016/j.jfluchem.2013.09.004.
Ronchin L, Vavasori A, Bortoluzzi M. Organocatalyzed Beckmann rearrangement of cyclohexanone oxime by trifluoroacetic acid in aprotic solvent. Catal Commun. 2008;10:251–6. https://doi.org/10.1016/j.catcom.2008.09.001.
Buchardt O, Jensen B. Photochemical studies XI. The photolysis of quinoxaline N-oxides to benz[d][1,3,6]-oxadiazepines. X-ray study Acta Chem Scand 1968;22:877–82.
Meanwell NA. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J Med Chem. 2018;61:5822–80. https://doi.org/10.1021/acs.jmedchem.7b01788.
Böhm H, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, et al. Fluorine in medicinal chemistry. ChemBiochem. 2004;5:637–43. https://doi.org/10.1002/cbic.200301023.
Yin Z, Li X, Deng Z, Yang Q, Peng Y. The synthesis of isoxazolo[2,3-c]quinazolines via a cycloaddition of quinazoline-3-oxides and acrylates. Tetrahedron Lett. 2020;61:151818 https://doi.org/10.1016/j.tetlet.2020.151818.
Wang Y, Liu Y, Yang Q, Mao X, Chai W-M, Peng Y. Study on the interaction between 4-(1H-indol-3-yl)-2-(p-tolyl)quinazoline-3- oxide and human serum albumin. Bioorg Med Chem. 2020;28:115720 https://doi.org/10.1016/j.bmc.2020.115720.
Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandin Leukot Essent Fatty Acids. 1998;58:17–24. https://doi.org/10.1016/S0952-3278(98)90125-9.
Liu B, Qu L, Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015;15:106 https://doi.org/10.1186/s12935-015-0260-7.
Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181 https://doi.org/10.1186/1471-2407-6-181.
Steele VE, Hawk ET, Viner JL, Kubet RA. Mechanisms and applications of the non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat Res. 2003;523:137–44. https://doi.org/10.1016/S0027-5107(02)00329-9.
Regulski M, Piotrowska-Kempisty H, Prukała W, Dutkiewicz Z, Regulska K, Stanisz B, et al. Synthesis, in vitro and in silico evaluation of novel trans-stilbene analogues as potential COX-2 inhibitors. Bioorg Med Chem. 2018;26:141–151. https://doi.org/10.1016/j.bmc.2017.11.027.
Michaux C, Charlier C. Structural approach for COX-2 inhibition. Mini-Rev Med Chem. 2004;4:603–15. https://doi.org/10.2174/1389557043403756.
Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644 https://doi.org/10.1038/384644a0.
Bruker S. Version 6.02 (Includes XPREP and SADABS). Madison, WI, USA: Bruker AXS Inc.; 2016.
Farrugia LJ. WinGX and ORTEP for Windows: an update. J Appl Crystallogr. 2012;45:849–54. https://doi.org/10.1107/S0021889812029111.
Sheldrick GM. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr A. 2015;71:3–8. https://doi.org/10.1107/S2053273314026370.
Sheldrick GM. Crystal structure refined with SHELXL. Acta Crystallogr C. 2015;71:3–8. https://doi.org/10.1107/S2053229614024218.
Spek AL. Structure validation in chemical crystallography. Acta Crystallogr D. 2009;65:148–55. https://doi.org/10.1107/S090744490804362X.
Nikkeri NM, Chikkur BP. Synthesis and antioxidant activity of 2-amino-5-methylthiazol derivatives containing 1,3,4-oxadiazole-2-thiol moiety. ISRN Org Chem. 2013;8:620718 https://doi.org/10.1155/2013/620718.
Morris V, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem. 2009;30:2785–91. https://doi.org/10.1002/jcc.21256.
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics. 2012;4:7 https://doi.org/10.1186/1758-2946-4-17.
Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M. PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015;43:W443–7. https://doi.org/10.1093/nar/gkv315.
Acknowledgements
The authors are grateful to the University of Stellenbosch Central Analytical Facility (CAF) and the University of the Witwatersrand for mass spectrometric and X-ray data, respectively.
Author contributions
Conceptualization and supervision, MJM and MMM; methodology, data curation and analyses, MJM; synthesis, EEO; bioassays, ENA; molecular docking, YSC; Writing: review & editing, MJM.
Funding
FundingThis project was funded by the University of South Africa, the National Research Foundation (NRF) in South Africa (NRF GUN: 118554), and the University Sains Malaysia (RUi grant: 1001/CIPPM/8011051). The views and opinions expressed in this article are those of the authors and not of the funding bodies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mphahlele, M.J., Onwu, E.E., Agbo, E.N. et al. Synthesis, in vitro and in silico enzyme (COX-1/2 & LOX-5), free radical scavenging and cytotoxicity profiling of the 2,4-dicarbo substituted quinazoline 3-oxides. Med Chem Res 31, 146–164 (2022). https://doi.org/10.1007/s00044-021-02811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02811-9