Abstract
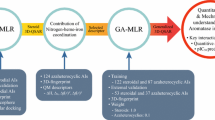
A QSAR analysis was conducted on a series of long-chained diarylalkylimidazole and diarylalkyltriazole derivatives as potent aromatase inhibitors. To obtain more appropriate QSAR models from a source of very large number of descriptors, a two-step stepwise variable selection strategy was performed. Firstly, from each group of the calculated descriptors, separate QSAR models were obtained. Then, the descriptors appeared in all of the generated models were subjected to another variable selection method and the obtained models were subjected to cross-validation. Finally, an external test set was used to access the ultimate performance of the models. The selected descriptors were analyzed for their influence on aromatase inhibition. The effects of hydration energy, position of H-bond acceptor, presence of cyano group, and shape of HOMO orbital on aromatase inhibition were successfully described, and they were consistent with the previous reports.





Similar content being viewed by others
References
Bayer H, Batzl C, Hartmann RW, Mannschreck A (1991) New aromatase inhibitors. Synthesis and biological activity of pyridyl-substituted tetralone derivatives. J Med Chem 34:2685–2691
Brodie AM, Njar VC (2000) Aromatase inhibitors and their application in breast cancer treatment. Steroids 65(4):171–179
Cavalli A, Recanatini M (2002) Looking for selectivity among cytochrome P450s inhibitors. J Med Chem 45:251–254
Doiron J, Soultan AH, Richard R, Touré MM, Picot N, Richard R, Čuperlović-Culf M, Robichaud GA, Touaibia M (2011) Synthesis and structure–activity relationship of 1- and 2-substituted-1,2,3-triazole letrozole-based analogues as aromatase inhibitors. Eur J Med Chem 46:4010–4024
Ferlin MG, Carta D, Bortolozzi B, Ghodsi R, Chimento A, Pezzi V, Moro S, Hanke N, Hartmann RW, Basso G, Viola G (2013) Design, synthesis, and structure–activity relationships of azolylmethylpyrroloquinolines as nonsteroidal aromatase inhibitors. J Med Chem 56:7536–7551
Garg R, Smith CJ (2014) Predicting the bioconcentration factor of highly hydrophobic organic chemicals. Food Chem Toxicol 69:252–259
Ghavami R, Sepehri B (2016) QSPR/QSAR solely based on molecular surface electrostatic potentials for benzenoid hydrocarbons. J Iran Chem Soc 13:519–529
Ghodsi R, Azizi E, Ferlin MG, Pezzi V, Zarghi A (2016) Design, synthesis and biological evaluation of 4-(Imidazolylmethyl)-2-aryl-quinoline derivatives as aromatase inhibitors and anti-breast cancer agents. Lett Drug Des Discov 13:89–97
Ghosh D, Griswold J, Erman M, Pangborn W (2010) X-ray structure of human aromatase reveals an androgen-specific active site. Steroid Biochem Mol Biol 118:197–202
Gobbi S, Zimmer C, Belluti F, Rampa A, Hartmann RW, Recanatini M, Bisi A (2010) Novel highly potent and selective nonsteroidal aromatase inhibitors: synthesis, biological evaluation and structure–activity relationships investigation. J Med Chem 53:5347–5351
Golbraikh A, Tropsha A (2002) Beware of q2. J Mol Graph Model 20:269–276
Hemmateenejad B, Yazdani M (2009) QSAR models for half-wave reduction potential of steroids: a comparative study between feature selection and feature extraction from subsets of or entire set of descriptors. Anal Chim Acta 634:27–35
Hong Y, Chen S (2006) Aromatase inhibitors: structural features and biochemical characterization. Ann N Y Acad Sci 1089:237–251
Jones CD, Winter MA, Hirsch KS, Stamm N, Talor HM, Holden HE, Davenport JD, Krumkalns EV, Suhr RG (1990) Estrogen synthetase inhibitors. 2. Comparison of the in vitro aromatase inhibitory activity for a variety of nitrogen heterocycles substituted with diarylmethane or diarylmethanol groups. J Med Chem 33:416–429
Karjalainen A, Pelkonen O, Sodervall ML, Lahde MA, Lammintausta RAS, Karjalainen AL, Kalapudas AM (1995) Aromatase inhibiting 4(5)-imidazoles. US Patent 5,439,928
Karjalainen A, Kalapudas A, Sodervall M, Pelkonen O, Lammintausta R (2000) Synthesis of new potent and selective aromatase inhibitors based on long-chained diarylalkylimidazole and diarylalkyltriazole molecule skeletons. Eur J Pharm Sci 11:109–131
Lang M, Batzl C, Furet P, Bowman R, Hausler A, Bhatnagar AS (1993) Structure–activity relationships and binding model of novel aromatase inhibitors. J Steroid Biochem Mol Biol 44:421–428
Leonetti F, Favia A, Rao A, Aliano R, Paluszcak A, Hartmann RW, Carotti A (2004) Design and synthesis, and 3D QSAR of novel potent and selective aromatase inhibitors. J Med Chem 47:6792–6803
McNulty J, Keskar K, Crankshaw DJ, Holloway A (2014) Discovery of a new class of cinnamyl-triazole as potent and selective inhibitors of aromatase (cytochrome P450 19A1). Bioorg Med Chem Lett 24:4586–4589
Mitra I, Saha A, Roy K (2010) Exploring quantitative structure–activity relationship studies of antioxidant phenolic compounds obtained from traditional Chinese medicinal plants. Mol Simul 36:1067–1079
Myint KZ, Xie XQ (2010) Recent advances in fragment-based QSAR and multi-dimensional QSAR methods. Int J Mol Sci 11:3846–3866
Needleman SJ, Tobias JS (2008) Aromatase inhibitors in early hormone receptor-positive breast cancer: what is the optimal initiation time for the maximum benefit? Drugs 68:1–15
Okada M, Yoden T, Kawaminami E, Shimada Y, Kudoh M, Isomura Y, Shikama H, Fujikura T (1996) Studies on aromatase inhibitors. I. Synthesis and biological evaluation of 4-amino-4H-1,2,4-triazole derivatives. Chem Pharm Bull 44:1871–1879
Okada M, Yoden T, Kawaminami E, Shimada Y, Kudoh M, Isomura Y (1997) Studies on aromatase inhibitors.II. Synthesis and biological evaluation of 1-amino-1H-1,2,4-triazole derivatives. Chem Pharm Bull 45:333–337
Pasha FA, Muddassar M, Beg Y, Cho SJ (2008) DFT-based de novo QSAR of phenoloxidase inhibitors. Chem Biol Drug Des 71:483–493
Pouget C, Yahiaoui S, Fagnere C, Habrioux G, Chulia AJ (2004) Synthesis and biological evaluation of 4-imidazolylflavans as nonsteroidal aromatase inhibitors. J Bioorg Chem 32:494–503
Recanatini M, Bisi A, Cavalli A, Belluti F, Gobbi S, Rampa A, Valenti P, Palzer M, Palusczak A, Hartmann RW (2001) A new class of nonsteroidal aromatase inhibitors: design and synthesis of chromone and xanthone derivatives and inhibition of the P450 enzymes aromatase and 17 alpha-hydroxylase/C17, 20-lyase. J Med Chem 44:672–680
Roy P, Roy K (2008) On some aspects of variable selection for partial least squares regression models. QSAR Comb Sci 27:302–313
Roy K, Kara S, Ambure P (2015) On a simple approach for determining applicability domain of QSAR models. Chemom Intell Lab Syst 145:22–29
Srivastava HK, Pasha FA, Mishra SK, Singh PP (2009) Novel applications of atomic softness and QSAR study of testosterone derivatives. Med Chem Res 18:455–466
Tropsha A, Gramatica P, Gombar VK (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSAR models. QSAR Comb Sci 22:69–77
Wong ZW, Ellis MJ (2004) First-line endocrine treatment of breast cancer. Br J Cancer 90:20–25
Yousefinejad S, Hemmateenejad B (2015) Chemometrics tools in QSAR/QSPR studies: a historical perspective. Chemom Intell Lab Syst 149:177–204
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghodsi, R., Hemmateenejad, B. QSAR study of diarylalkylimidazole and diarylalkyltriazole aromatase inhibitors. Med Chem Res 25, 834–842 (2016). https://doi.org/10.1007/s00044-016-1530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1530-1