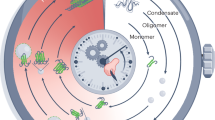
Abstract
The development of aging is associated with the disruption of key cellular processes manifested as well-established hallmarks of aging. Intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs) have no stable tertiary structure that provide them a power to be configurable hubs in signaling cascades and regulate many processes, potentially including those related to aging. There is a need to clarify the roles of IDPs/IDRs in aging. The dataset of 1702 aging-related proteins was collected from established aging databases and experimental studies. There is a noticeable presence of IDPs/IDRs, accounting for about 36% of the aging-related dataset, which is however less than the disorder content of the whole human proteome (about 40%). A Gene Ontology analysis of the used here aging proteome reveals an abundance of IDPs/IDRs in one-third of aging-associated processes, especially in genome regulation. Signaling pathways associated with aging also contain IDPs/IDRs on different hierarchical levels, revealing the importance of "structure-function continuum" in aging. Protein–protein interaction network analysis showed that IDPs present in different clusters associated with different aging hallmarks. Protein cluster with IDPs enrichment has simultaneously high liquid–liquid phase separation (LLPS) probability, “nuclear” localization and DNA-associated functions, related to aging hallmarks: genomic instability, telomere attrition, epigenetic alterations, and stem cells exhaustion. Intrinsic disorder, LLPS, and aggregation propensity should be considered as features that could be markers of pathogenic proteins. Overall, our analyses indicate that IDPs/IDRs play significant roles in aging-associated processes, particularly in the regulation of DNA functioning. IDP aggregation, which can lead to loss of function and toxicity, could be critically harmful to the cell. A structure-based analysis of aging and the identification of proteins that are particularly susceptible to disturbances can enhance our understanding of the molecular mechanisms of aging and open up new avenues for slowing it down.












Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and also present as electronic supplementary materials. Additional data will be available on reasonable request.
Change history
13 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00018-023-04989-0
Abbreviations
- IDP:
-
Intrinsically disordered protein
- IDR:
-
Intrinsically disordered region
- PONDR:
-
Predictor of natural disordered regions
- PONDR-FIT, VLXT, VL3, VSL2, IUPred2A:
-
Predictors of protein disorder (existence of the disordered residues in the protein) from PONDR family
- MDS:
-
Mean disorder score—average of PONDR scores of each residue for whole protein
- PPIDR:
-
Predicted percentage of intrinsically disordered residues (with propensity to be disordered higher than 0.5)
- CDF:
-
Cumulative distribution function
- CH:
-
Charge–hydropathy
- MoRF:
-
Molecular recognition features (protein–partner interaction sites that acquire structure upon binding to partners)
- LLPS:
-
Liquid–liquid phase separation
- FuzDrop, PSPredictor, PScore:
-
Predictors of the LLPS propensity
- PPI:
-
Protein–protein interaction
- BP:
-
Biological process
- AggreScan, PASTA2:
-
Predictors of the aggregation propensity
- IIS:
-
Insulin and insulin-like signaling
- DR:
-
Dietary restriction
- TERT:
-
Telomerase catalytic unit
- nFoE:
-
Normalized fold enrichment
- ML:
-
Machine learning
References
López-Otín C, Blasco MA, Partridge L, et al (2013) The hallmarks of aging. Cell 153:1194–1217
López-Otín C, Blasco MA, Partridge L, et al (2023) Hallmarks of aging: An expanding universe. Cell 186:243–278
Anisimov VN (2003) Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Exp Gerontol 38:1041–1049
Franceschi C, Garagnani P, Morsiani C et al (2018) The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 5:61
Wright PE, Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16:18–29
Dunker AK, Silman I, Uversky VN, Sussman JL (2008) Function and structure of inherently disordered proteins. Curr Opin Struct Biol 18:756–764
Uversky VN (2013) Intrinsic disorder-based protein interactions and their modulators. Curr Pharm Des 19:4191–4213
Uversky VN (2013) Unusual biophysics of intrinsically disordered proteins. Biochim Biophys Acta (BBA) Proteins Proteom 1834:932–951
Uversky VN (2015) Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J 282:1182–1189
Dunker AK, Cortese MS, Romero P et al (2005) Flexible nets: the roles of intrinsic disorder in protein interaction networks. FEBS J 272:5129–5148
Bondos SE, Dunker AK, Uversky VN (2021) On the roles of intrinsically disordered proteins and regions in cell communication and signaling. Cell Commun Signal 19:88
Uversky VN, Oldfield CJ, Dunker AK (2005) Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit: Interdiscip J 18:343–384
Van Der Lee R, Buljan M, Lang B et al (2014) Classification of intrinsically disordered regions and proteins. Chem Rev 114:6589–6631
Schulz GE (1979) Nucleotide binding proteins. In: Molecular mechanisms of biological recognition. Elsevier, Amsterdam, pp 79–94
Dunker AK, Obradovic Z (2001) The protein trinity—linking function and disorder. Nat Biotechnol 19:805–806
Uversky VN, Dunker AK (2010) Understanding protein non-folding. Biochim Biophys Acta (BBA) Proteins Proteom 1804:1231–1264
Oldfield CJ, Cheng Y, Cortese MS et al (2005) Coupled folding and binding with α-helix-forming molecular recognition elements. Biochemistry 44:12454–12470
Mohan A, Oldfield CJ, Radivojac P et al (2006) Analysis of molecular recognition features (MoRFs). J Mol Biol 362:1043–1059
Vacic V, Oldfield CJ, Mohan A et al (2007) Characterization of molecular recognition features, MoRFs, and their binding partners. J Proteome Res 6:2351–2366
Cheng Y, Oldfield CJ, Meng J et al (2007) Mining α-helix-forming molecular recognition features with cross species sequence alignments. Biochemistry 46:13468–13477
Disfani FM, Hsu W-L, Mizianty MJ et al (2012) MoRFpred, a computational tool for sequence-based prediction and characterization of short disorder-to-order transitioning binding regions in proteins. Bioinformatics 28:i75–i83
Yan J, Dunker AK, Uversky VN, Kurgan L (2016) Molecular recognition features (MoRFs) in three domains of life. Mol Biosyst 12:697–710
Mészáros B, Simon I, Dosztányi Z (2011) The expanding view of protein–protein interactions: complexes involving intrinsically disordered proteins. Phys Biol 8:035003
Malaney P, Pathak RR, Xue B et al (2013) Intrinsic disorder in PTEN and its interactome confers structural plasticity and functional versatility. Sci Rep 3:1–14
Koshland DE Jr (1958) Application of a theory of enzyme specificity to protein synthesis. Proc Natl Acad Sci 44:98–104
Uversky VN (2019) Protein intrinsic disorder and structure-function continuum. Prog Mol Biol Transl Sci 166:1–17
Fonin AV, Darling AL, Kuznetsova IM et al (2019) Multi-functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder-based proteoforms. Cell Mol Life Sci 76:4461–4492
Uversky VN (2016) p53 proteoforms and intrinsic disorder: an illustration of the protein structure–function continuum concept. Int J Mol Sci 17:1874
Uversky VN (2016) Dancing protein clouds: the strange biology and chaotic physics of intrinsically disordered proteins. J Biol Chem 291:6681–6688
Nesterov SV, Ilyinsky NS, Uversky VN (2021) Liquid–liquid phase separation as a common organizing principle of intracellular space and biomembranes providing dynamic adaptive responses. Biochim Biophys Acta (BBA) Mol Cell Res 1868:119102
Cermakova K, Demeulemeester J, Lux V, et al (2021) A ubiquitous disordered protein interaction module orchestrates transcription elongation. Science 374: 1113–1121
Cermakova K, Veverka V, Hodges HC (2023) The TFIIS N-terminal domain (TND): a transcription assembly module at the interface of order and disorder. Biochem Soc Trans 51:125–135
Uversky VN, Kuznetsova IM, Turoverov KK, Zaslavsky B (2015) Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Lett 589:15–22
Meng F, Na I, Kurgan L, Uversky VN (2016) Compartmentalization and functionality of nuclear disorder: intrinsic disorder and protein–protein interactions in intra-nuclear compartments. Int J Mol Sci 17:24. https://doi.org/10.3390/ijms17010024
Uversky VN (2017) Protein intrinsic disorder-based liquid–liquid phase transitions in biological systems: complex coacervates and membrane-less organelles. Adv Colloid Interface Sci 239:97–114
Uversky VN (2017) Intrinsically disordered proteins in overcrowded milieu: membrane-less organelles, phase separation, and intrinsic disorder. Curr Opin Struct Biol 44:18–30
Darling AL, Liu Y, Oldfield CJ, Uversky VN (2018) Intrinsically disordered proteome of human membrane-less organelles. Proteomics 18:1700193
Darling AL, Zaslavsky BY, Uversky VN (2019) Intrinsic disorder-based emergence in cellular biology: physiological and pathological liquid-liquid phase transitions in cells. Polymers (Basel) 11:990
Turoverov KK, Kuznetsova IM, Fonin AV et al (2019) Stochasticity of biological soft matter: emerging concepts in intrinsically disordered proteins and biological phase separation. Trends Biochem Sci 44:716–728
Uversky VN (2019) Supramolecular fuzziness of intracellular liquid droplets: liquid–liquid phase transitions, membrane-less organelles, and intrinsic disorder. Molecules 24:3265
Uversky VN (2021) Recent developments in the field of intrinsically disordered proteins: intrinsic disorder-based emergence in cellular biology in light of the physiological and pathological liquid–liquid phase transitions. Annu Rev Biophys 50:135–156
Kulkarni P, Bhattacharya S, Achuthan S et al (2022) Intrinsically disordered proteins: critical components of the wetware. Chem Rev 122:6614–6633
Antifeeva IA, Fonin AV, Fefilova AS et al (2022) Liquid–liquid phase separation as an organizing principle of intracellular space: overview of the evolution of the cell compartmentalization concept. Cell Mol Life Sci 79:251
Fonin AV, Antifeeva IA, Kuznetsova IM et al (2022) Biological soft matter: intrinsically disordered proteins in liquid–liquid phase separation and biomolecular condensates. Essays Biochem 66:831–847
Lyons H, Veettil RT, Pradhan P et al (2023) Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 186:327-345.e28. https://doi.org/10.1016/j.cell.2022.12.013
Dunker AK, Romero P, Obradovic Z et al (2000) Intrinsic protein disorder in complete genomes. Genome Inform 11:161–171
Ward JJ, Sodhi JS, McGuffin LJ et al (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337:635–645
Xue B, Dunker AK, Uversky VN (2012) Orderly order in protein intrinsic disorder distribution: disorder in 3500 proteomes from viruses and the three domains of life. J Biomol Struct Dyn 30:137–149
Kim J-Y, Cho Y-E, Park J-H (2015) The nucleolar protein GLTSCR2 is an upstream negative regulator of the oncogenic nucleophosmin-MYC axis. Am J Pathol 185:2061–2068
Shi Y, Xu X, Zhang Q et al (2014) tRNA synthetase counteracts c-Myc to develop functional vasculature. Elife 3:e02349
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Wu K-J, Grandori C, Amacker M et al (1999) Direct activation of TERT transcription by c-MYC. Nat Genet 21:220–224
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Ocampo A, Reddy P, Martinez-Redondo P et al (2016) In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167:1719–1733
Lu Y, Brommer B, Tian X et al (2020) Reprogramming to recover youthful epigenetic information and restore vision. Nature 588:124–129
Yang J-H, Hayano M, Griffin PT, et al (2023) Loss of epigenetic information as a cause of mammalian aging. Cell 186:305–326
Xue B, Dunbrack RL, Williams RW et al (2010) PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim Biophys Acta (BBA) Proteins Proteom 1804:996–1010
Akdel M, Pires DEV, Pardo EP et al (2022) A structural biology community assessment of AlphaFold2 applications. Nat Struct Mol Biol 29:1056–1067. https://doi.org/10.1038/s41594-022-00849-w
Zhao B, Ghadermarzi S, Kurgan L (2023) Comparative evaluation of AlphaFold2 and disorder predictors for prediction of intrinsic disorder, disorder content and fully disordered proteins. Comput Struct Biotechnol J 21:3248–3258. https://doi.org/10.1016/j.csbj.2023.06.001
Piovesan D, Monzon AM, Tosatto SCE (2022) Intrinsic protein disorder and conditional folding in AlphaFoldDB. Protein Sci. https://doi.org/10.1002/pro.4466
Kovacs D, Tompa P (2012) Diverse functional manifestations of intrinsic structural disorder in molecular chaperones. Biochem Soc Trans 40(5):963–968. https://doi.org/10.1042/BST20120108
Lindner AB, Demarez A (2009) Protein aggregation as a paradigm of aging. Biochim Biophys Acta 1790(10):980–996. https://doi.org/10.1016/j.bbagen.2009.06.005
Csermely P, Sőti C (2006) Cellular networks and the aging process. Arch Physiol Biochem 112(2):60–64. https://doi.org/10.1080/13813450600711243
Iakoucheva LM, Radivojac P, Brown CJ et al (2004) The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res 32:1037–1049
Collins MO, Yu L, Campuzano I et al (2008) Phosphoproteomic analysis of the mouse brain cytosol reveals a predominance of protein phosphorylation in regions of intrinsic sequence disorder. Mol Cell Proteom 7:1331–1348
Darling AL, Uversky VN (2018) Intrinsic disorder and posttranslational modifications: the darker side of the biological dark matter. Front Genet 9:158
Pejaver V, Hsu W, Xin F et al (2014) The structural and functional signatures of proteins that undergo multiple events of post-translational modification. Protein Sci 23:1077–1093
Xie H, Vucetic S, Iakoucheva LM et al (2007) Functional anthology of intrinsic disorder. 3. Ligands, post-translational modifications, and diseases associated with intrinsically disordered proteins. J Proteome Res 6:1917–1932
Smith NC, Kuravsky M, Shammas SL, Matthews JM (2021) Binding and folding in transcriptional complexes. Current Opin Struct Biol 66:156–162. https://doi.org/10.1016/j.sbi.2020.10.026
Gian Carlo GP, Ivette P, Jennifer LF, Britney NH, Lee H-W, Carrie LP (2020) The human CRY1 tail controls circadian timing by regulating its association with CLOCK:BMAL1 Significance Proceedings of the National Academy of Sciences 117(45):27971–27979. https://doi.org/10.1073/pnas.1920653117
Gustafson CL, Parsley NC, Asimgil H, et al (2017) A Slow Conformational Switch in the BMAL1 Transactivation Domain Modulates Circadian Rhythms. Mol Cell 66(4):447–457.e7. https://doi.org/10.1016/j.molcel.2017.04.011
Partch CL (2020) Orchestration of Circadian Timing by Macromolecular Protein Assemblies. J Mol Biol 432(12):3426–3448. https://doi.org/10.1016/j.jmb.2019.12.046
Vinogradova IA, Anisimov VN, Bukalev AV, et al (2009) Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats. Aging 1(10):855–865. https://doi.org/10.18632/aging.v1i1010.18632/aging.100092
Riera CE, Dillin A (2015) Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol 17(3):196–203. https://doi.org/10.1038/ncb3107
Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E (2019) From discoveries in ageing research to therapeutics for healthy ageing. Nature 571(7764):183–192. https://doi.org/10.1038/s41586-019-1365-2
Uversky VN, Oldfield CJ, Midic U, et al (2009) Unfoldomics of human diseases: linking protein intrinsic disorder with diseases BMC Genomics 10(Suppl 1): S7. https://doi.org/10.1186/1471-2164-10-S1-S7
Joerger AC, Fersht AR (2007) Structure–function–rescue: the diverse nature of common p53 cancer mutants. Oncogene 26(15) :2226–2242 https://doi.org/10.1038/sj.onc.1210291
Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO reports 15(11):1139–1153. https://doi.org/10.15252/embr.201439245
Ilyinsky NS, Nesterov SV, Shestoperova EI et al (2021) On the role of normal aging processes in the onset and pathogenesis of diseases associated with the abnormal accumulation of protein aggregates. Biochem Mosc 86:275–289
Uversky VN (2015) Intrinsically disordered proteins and their (disordered) proteomes in neurodegenerative disorders. Front Aging Neurosci 7:18
Gadhave K, Gehi BR, Kumar P et al (2020) Subclassifying disordered proteins by the CH-CDF plot method. Cell Mol Life Sci 77:4163–4208
Uversky VN (2017) The roles of intrinsic disorder-based liquid-liquid phase transitions in the “Dr. Jekyll–Mr. Hyde” behavior of proteins involved in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Autophagy 13:2115–2162
Iakoucheva LM, Brown CJ, Lawson JD et al (2002) Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol 323:573–584
Uversky VN (2008) Amyloidogenesis of natively unfolded proteins. Curr Alzheimer Res 5:260–287
Cheng Y, LeGall T, Oldfield CJ et al (2006) Abundance of intrinsic disorder in protein associated with cardiovascular disease. Biochemistry 45:10448–10460
Du Z, Uversky VN (2017) A comprehensive survey of the roles of highly disordered proteins in type 2 diabetes. Int J Mol Sci 18:2010
Bolognesi B, Faure AJ, Seuma M et al (2019) The mutational landscape of a prion-like domain. Nat Commun 10:1–12
Miller SBM, Mogk A, Bukau B (2015) Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J Mol Biol 427:1564–1574
Li J, McQuade T, Siemer AB et al (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350. https://doi.org/10.1016/j.cell.2012.06.019
Garcia-Pardo J, Bartolomé-Nafría A, Chaves-Sanjuan A et al (2023) Cryo-EM structure of hnRNPDL-2 fibrils, a functional amyloid associated with limb-girdle muscular dystrophy D3. Nat Commun. https://doi.org/10.1038/s41467-023-35854-0
Rawat P, Prabakaran R, Sakthivel R et al (2020) CPAD 2.0: a repository of curated experimental data on aggregating proteins and peptides. Amyloid 27:128–133. https://doi.org/10.1080/13506129.2020.1715363
Darling AL, Shorter J (2021) Combating deleterious phase transitions in neurodegenerative disease. Biochim Biophys Acta (BBA) Mol Cell Res 1868:118984
Alberti S, Hyman AA (2016) Are aberrant phase transitions a driver of cellular aging? BioEssays 38:959–968. https://doi.org/10.1002/bies.201600042
Garaizar A, Espinosa JR, Joseph JA, et al (2022) Aging can transform single-component protein condensates into multiphase architectures. Proc Nat Acad Sci 104: 5925–5930 https://doi.org/10.1073/pnas
Guillén-Boixet J, Kopach A, Holehouse AS et al (2020) RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181:346-361.e17. https://doi.org/10.1016/j.cell.2020.03.049
Lechler MC, David DC (2017) More stressed out with age? Check your RNA granule aggregation. Prion 11:313–322
Verdile V, De Paola E, Paronetto MP (2019) Aberrant Phase Transitions: Side Effects and Novel Therapeutic Strategies in Human Disease. Front Genet 10:173
Shiina N (2019) Liquid- and solid-like RNA granules form through specific scaffold proteins and combine into biphasic granules. J Biol Chem 294:3532–3548. https://doi.org/10.1074/jbc.RA118.005423
Tüű-Szabó B, Hoffka G, Duro N, Fuxreiter M (2019) Altered dynamics may drift pathological fibrillization in membraneless organelles. Biochim Biophys Acta Proteins Proteom 1867:988–998. https://doi.org/10.1016/j.bbapap.2019.04.005
Cao X, Jin X, Liu B (2020) The involvement of stress granules in aging and aging-associated diseases. Aging Cell 19:e13136
Maharjan N, Künzli C, Buthey K, Saxena S (2017) C9ORF72 regulates stress granule formation and its deficiency impairs stress granule assembly, hypersensitizing cells to stress. Mol Neurobiol 54:3062–3077. https://doi.org/10.1007/s12035-016-9850-1
Chitiprolu M, Jagow C, Tremblay V et al (2018) A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy. Nat Commun. https://doi.org/10.1038/s41467-018-05273-7
Lechler MC, Crawford ED, Groh N et al (2017) Reduced insulin/IGF-1 signaling restores the dynamic properties of key stress granule proteins during aging. Cell Rep 18:454–467. https://doi.org/10.1016/j.celrep.2016.12.033
Portz B, Lee BL, Shorter J (2021) FUS and TDP-43 phases in health and disease. Trends Biochem Sci 46:550–563
Heinze I, Bens M, Calzia E et al (2018) Species comparison of liver proteomes reveals links to naked mole-rat longevity and human aging. BMC Biol. https://doi.org/10.1186/s12915-018-0547-y
Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, et al (2019) Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife 8:e49874. https://doi.org/10.7554/eLife.49874.001
Jumper J, Evans R, Pritzel A et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. https://doi.org/10.1038/s41586-021-03819-2
Altschup SF, Gish W, Miller W, et al (1990) Basic Local Alignment Search Tool. J Mol Biol 215:403–410.
Pettersen EF, Goddard TD, Huang CC et al (2021) UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30:70–82. https://doi.org/10.1002/pro.3943
Tacutu R, Thornton D, Johnson E et al (2018) Human ageing genomic resources: new and updated databases. Nucleic Acids Res 46:D1083–D1090
Ashburner M, Ball CA, Blake JA et al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29
The Gene Ontology Consortium (2021) The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res 49:D325–D334
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Craig T, Smelick C, Tacutu R et al (2015) The Digital Ageing Atlas: integrating the diversity of age-related changes into a unified resource. Nucleic Acids Res 43:D873–D878. https://doi.org/10.1093/nar/gku843
Liu GH, Bao Y, Qu J et al (2021) Aging Atlas: a multi-omics database for aging biology. Nucleic Acids Res 49:D825–D830. https://doi.org/10.1093/nar/gkaa894
Hühne R, Thalheim T, Sühnel J (2014) AgeFactDB—The JenAge Ageing Factor Database—towards data integration in ageing research. Nucleic Acids Res. https://doi.org/10.1093/nar/gkt1073
Johnson AA, Shokhirev MN, Wyss-Coray T, Lehallier B (2020) Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res Rev 60:101070
Peters MJ, Joehanes R, Pilling LC et al (2015) The transcriptional landscape of age in human peripheral blood. Nat Commun 6:1–14
Lu T, Pan Y, Kao S-Y et al (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883–891
Brehme M, Voisine C, Rolland T et al (2014) A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep 9:1135–1150
Zeng YI, Nie C, Min J et al (2016) Novel loci and pathways significantly associated with longevity. Sci Rep 6:1–13
Apweiler R, Bairoch A, Wu CH et al (2004) UniProt: the universal protein knowledgebase. Nucleic Acids Res 32:D115–D119
Kerepesi C, Daróczy B, Sturm Á et al (2018) Prediction and characterization of human ageing-related proteins by using machine learning. Sci Rep 8:1–13
Dayhoff GW, Uversky VN (2022) Rapid prediction and analysis of protein intrinsic disorder. Protein Sci 31:e4496
Li X, Romero P, Rani M et al (1999) Predicting protein disorder for N-, C-and internal regions. Genome Inform 10:30–40
Peng K, Radivojac P, Vucetic S et al (2006) Length-dependent prediction of protein intrinsic disorder. BMC Bioinform 7:1–17
Radivojac P, Obradović Z, Brown CJ, Dunker AK (2002) Prediction of boundaries between intrinsically ordered and disordered protein regions. In: Biocomputing 2003. World Scientific, Singapore, pp 216–227
Dosztanyi Z, Csizmok V, Tompa P, Simon I (2005) The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 347:827–839
Huang F, Oldfield C, Meng J et al (2012) Subclassifying disordered proteins by the CH-CDF plot method. In: Biocomputing 2012. World Scientific, Singapore, pp 128–139
van Bibber NW, Haerle C, Khalife R et al (2020) Intrinsic disorder in tetratricopeptide repeat proteins. Int J Mol Sci 21:3709
Uversky VN, Gillespie JR, Fink AL (2000) Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins: Struct Funct Bioinform 41:415–427
Oldfield CJ, Cheng Y, Cortese MS et al (2005) Comparing and combining predictors of mostly disordered proteins. Biochemistry 44:1989–2000
Mohan A, Sullivan WJ Jr, Radivojac P et al (2008) Intrinsic disorder in pathogenic and non-pathogenic microbes: discovering and analyzing the unfoldomes of early-branching eukaryotes. Mol Biosyst 4:328–340
Huang F, Oldfield CJ, Xue B et al (2014) Improving protein order-disorder classification using charge-hydropathy plots. BMC Bioinform 15:1–13
Dosztányi Z, Mészáros B, Simon I (2009) ANCHOR: web server for predicting protein binding regions in disordered proteins. Bioinformatics 25:2745–2746
Oates ME, Romero P, Ishida T et al (2012) D2P2: database of disordered protein predictions. Nucleic Acids Res 41:D508–D516
Conchillo-Solé O, de Groot NS, Avilés FX et al (2007) AGGRESCAN: a server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinform 8:1–17
de Groot NS, Pallarés I, Avilés FX et al (2005) Prediction of “hot spots” of aggregation in disease-linked polypeptides. BMC Struct Biol 5:1–15
Walsh I, Seno F, Tosatto SCE, Trovato A (2014) PASTA 2.0: an improved server for protein aggregation prediction. Nucleic Acids Res. https://doi.org/10.1093/nar/gku399
Chu X, Sun T, Li Q et al (2022) Prediction of liquid–liquid phase separating proteins using machine learning. BMC Bioinform 23:1–13
Hardenberg M, Horvath A, Ambrus V et al (2020) Widespread occurrence of the droplet state of proteins in the human proteome. Proc Natl Acad Sci 117:33254–33262
Vendruscolo M, Fuxreiter M (2022) Sequence determinants of the aggregation of proteins within condensates generated by liquid–liquid phase separation. J Mol Biol 434:167201
Hatos A, Tosatto SCE, Vendruscolo M, Fuxreiter M (2022) FuzDrop on AlphaFold: visualizing the sequence-dependent propensity of liquid–liquid phase separation and aggregation of proteins. Nucleic Acids Res 50:W337–W344
McCoy Vernon R, Andrew Chong P, Tsang B, et al (2018) Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7: e31486. https://doi.org/10.7554/eLife.31486.001
Thomas PD, Campbell MJ, Kejariwal A et al (2003) PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13:2129–2141
Shannon P, Markiel A, Ozier O et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Szklarczyk D, Gable AL, Lyon D et al (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613
Maere S, Heymans K, Kuiper M (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21:3448–3449
Uversky VN (2003) A protein-chameleon: conformational plasticity of α-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn 21:211–234
Uversky VN (2010) Targeting intrinsically disordered proteins in neurodegenerative and protein dysfunction diseases: another illustration of the D2 concept. Expert Rev Proteom 7:543–564
Uversky VN (2008) α-Synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci 9:507–540
Uversky VN (2009) Intrinsic disorder in proteins associated with neurodegenerative diseases. In: Ovádi, J., Orosz, F. (eds) Protein Folding and Misfolding: Neurodegenerative Diseases. Focus on Structural Biology, vol 7. Springer, Dordrecht, pp 21–75
Uversky VN, Oldfield CJ, Dunker AK (2008) Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys 37:215–246
Breydo L, Wu JW, Uversky VN (2012) α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta (BBA) Mol Basis Dis 1822:261–285
Uversky VN (2014) The triple power of D (3): protein intrinsic disorder in degenerative diseases. Front Biosci (Landmark Ed) 19:181–258
Uversky VN (2017) Looking at the recent advances in understanding α-synuclein and its aggregation through the proteoform prism. F1000Res 6:525. https://doi.org/10.12688/f1000research.10536.1
Coskuner-Weber O, Mirzanli O, Uversky VN (2022) Intrinsically disordered proteins and proteins with intrinsically disordered regions in neurodegenerative diseases. Biophys Rev 14:679–707
DeForte S, Uversky VN (2017) Not an exception to the rule: the functional significance of intrinsically disordered protein regions in enzymes. Mol Biosyst 13:463–469. https://doi.org/10.1039/C6MB00741D
Haynes C, Oldfield CJ, Ji F et al (2006) Intrinsic disorder is a common feature of hub proteins from four eukaryotic interactomes. PLoS Comput Biol 2:e100
Lehallier B, Gate D, Schaum N et al (2019) Undulating changes in human plasma proteome profiles across the lifespan. Nat Med 25:1843–1850
de Cabo R, Carmona-Gutierrez D, Bernier M et al (2014) The search for antiaging interventions: from elixirs to fasting regimens. Cell 157:1515–1526
Cermakova K, Hodges HC (2023) Interaction modules that impart specificity to disordered protein. Trends Biochem Sci 5:477–490
Wang C, Uversky VN, Kurgan L (2016) Disordered nucleiome: abundance of intrinsic disorder in the DNA- and RNA-binding proteins in 1121 species from Eukaryota, Bacteria and Archaea. Proteomics 16:1486–1498. https://doi.org/10.1002/pmic.201500177
Zhao B, Katuwawala A, Uversky VN, Kurgan L (2021) IDPology of the living cell: intrinsic disorder in the subcellular compartments of the human cell. Cell Mol Life Sci 78:2371–2385. https://doi.org/10.1007/s00018-020-03654-0
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Babinchak WM, Surewicz WK (2020) Liquid–liquid phase separation and its mechanistic role in pathological protein aggregation. J Mol Biol 432:1910–1925
Ahmad A, Uversky VN, Khan RH (2022) Aberrant liquid–liquid phase separation and amyloid aggregation of proteins related to neurodegenerative diseases. Int J Biol Macromol 220:703–720
Tartaglia GG, Vendruscolo M (2010) Proteome-level interplay between folding and aggregation propensities of proteins. J Mol Biol 402:919–928
Statello L, Guo C-J, Chen L-L, Huarte M (2021) Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 22:96–118
Gadhave K, Kumar D, Uversky VN, Giri R (2021) A multitude of signaling pathways associated with Alzheimer’s disease and their roles in AD pathogenesis and therapy. Med Res Rev 41:2689–2745
Coskuner O, Uversky VN (2019) Intrinsically disordered proteins in various hypotheses on the pathogenesis of Alzheimer’s and Parkinson’s diseases. Prog Mol Biol Transl Sci 166:145–223
Salahuddin P, Fatima MT, Uversky VN et al (2021) The role of amyloids in Alzheimer’s and Parkinson’s diseases. Int J Biol Macromol 190:44–55
Darling AL, Breydo L, Rivas EG et al (2019) Repeated repeat problems: combinatorial effect of C9orf72-derived dipeptide repeat proteins. Int J Biol Macromol 127:136–145
Tejedor AR, Sanchez-Burgos I, Estevez-Espinosa M et al (2022) Protein structural transitions critically transform the network connectivity and viscoelasticity of RNA-binding protein condensates but RNA can prevent it. Nat Commun. https://doi.org/10.1038/s41467-022-32874-0
Ganne A, Balasubramaniam M, Ayyadevara S, Shmookler Reis RJ (2022) Machine-learning analysis of intrinsically disordered proteins identifies key factors that contribute to neurodegeneration-related aggregation. Front Aging Neurosci 14:938117
Mukherjee P, Panda P, Kasturi P (2022) A comparative meta-analysis of membraneless organelle-associated proteins with age related proteome of C. elegans. Cell Stress Chaperones 27:619–631
Acknowledgements
NI acknowledges the Ministry of Science and Higher Education of the Russian Federation (Agreement # 075-03-2023-106, Project FSMG-2020-0003, in the part of analysis of protein-protein interactions. The research was also supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement # 075-01593-23-04, Project 720000F.99.1.BN62AA40000) in the part of aggregation evaluation. The part of this work (LLPS analysis) was funded by Russian Science Foundation, Grant number 21-75-10166 (A.V.F.).
Funding
This work was supported in part by the Ministry of Science and Higher Education of the Russian Federation (Agreement # 075-03-2023-106, Project FSMG-2020-0003; and Agreement # 075-01593-23-04, Project 720000F.99.1.BN62AA40000). The part of this work was funded by Russian Science Foundation (Grant number 21-75-10166).
Author information
Authors and Affiliations
Contributions
VDM and NSI contributed equally to this work. NSI, VI, and VNU conceived and designed study. VDM, NSI, SVN, BMGAS, GWD, EVZ, SSM, AVF, IMK, KKT, and VNU conducted research, analyzed data, and collected and analyzed literature data. VDM, NSI, SVN, BMGAS, GWD, EVZ, and SSM designed illustrations. VDM, NSI, SVN, BMGAS, GWD, EVZ, SSM, AVF, IMK, KKT, VI, and VNU wrote the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manyilov, V.D., Ilyinsky, N.S., Nesterov, S.V. et al. Chaotic aging: intrinsically disordered proteins in aging-related processes. Cell. Mol. Life Sci. 80, 269 (2023). https://doi.org/10.1007/s00018-023-04897-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04897-3