Abstract
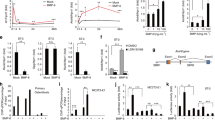
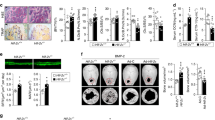
Imbalance of bone homeostasis induces bone degenerative diseases such as osteoporosis. Hedgehog (Hh) signaling plays critical roles in regulating the development of limb and joint. However, its unique role in bone homeostasis remained largely unknown. Here, we found that canonical Hh signaling pathway was gradually augmented during osteoclast differentiation. Genetic inactivation of Hh signaling in osteoclasts, using Ctsk-Cre;Smof/f conditional knockout mice, disrupted both osteoclast formation and subsequent osteoclast–osteoblast coupling. Concordantly, either Hh signaling inhibitors or Smo/Gli2 knockdown stunted in vitro osteoclast formation. Mechanistically, Hh signaling positively regulated osteoclast differentiation via transactivation of Traf6 and stabilization of TRAF6 protein. Then, we identified connective tissue growth factor (CTGF) as an Hh-regulatory bone formation-stimulating factor derived from osteoclasts, whose loss played a causative role in osteopenia seen in CKO mice. In line with this, recombinant CTGF exerted mitigating effects against ovariectomy induced bone loss, supporting a potential extension of local rCTGF treatment to osteoporotic diseases. Collectively, our findings firstly demonstrate that Hh signaling, which dictates osteoclast differentiation and osteoclast–osteoblast coupling by regulating TRAF6 and CTGF, is crucial for maintaining bone homeostasis, shedding mechanistic and therapeutic insights into the realm of osteoporosis.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author LY upon reasonable request.
References
Crane J, Cao X (2014) Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Investig 124(2):466–472
Kalinkovich A, Livshits G (2021) Biased and allosteric modulation of bone cell-expressing g protein-coupled receptors as a novel approach to osteoporosis therapy. Pharmacol Res 171:105794
Rachner T, Khosla S, Hofbauer L (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Bar-Shavit Z (2007) The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem 102(5):1130–1139
Kim J, Kim N (2016) Signaling pathways in osteoclast differentiation. Chonnam Med J 52(1):12–17
Mun SH, Park P, Park-Min KH (2020) The m-csf receptor in osteoclasts and beyond. Exp Mol Med 52(8):1239–1254
Sobacchi C, Schulz A, Coxon F, Villa A, Helfrich M (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9(9):522–536
Makras P, Delaroudis S, Anastasilakis AD (2015) Novel therapies for osteoporosis. Metabolism 64(10):1199–1214
Yang J, Andre P, Ye L, Yang Y (2015) The hedgehog signalling pathway in bone formation. Int J Oral Sci 7(2):73–79
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23(7):1128–1139
Alman B (2015) The role of hedgehog signalling in skeletal health and disease. Nat Rev Rheumatol 11(9):552–560
Lanske B, Karaplis A, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize L, Ho C, Mulligan R, Abou-Samra A, Juppner H, Segre G, Kronenberg H (1996) Pth/pthrp receptor in early development and Indian hedgehog-regulated bone growth. Science 273(5275):663–666
Wang H, Zheng C, Lu W, He T, Fan J, Wang C, Jie Q, Chan D, Cheah K, Yang L (2022) Hedgehog signaling orchestrates cartilage-to-bone transition independently of smoothened. Matrix Biol 110:76–90
Zhang L, Fu X, Ni L, Liu C, Zheng Y, You H, Li M, Xiu C, Zhang L, Gong T, Luo N, Zhang Z, He G, Hu S, Yang H, Chen D, Chen J (2022) Hedgehog signaling controls bone homeostasis by regulating osteogenic/adipogenic fate of skeletal stem/progenitor cells in mice. J Bone Miner Res 37(3):559–576
Mak K, Bi Y, Wan C, Chuang P, Clemens T, Young M, Yang Y (2008) Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling pthrp and rankl expression. Dev Cell 14(5):674–688
Shimo T, Matsumoto K, Takabatake K, Aoyama E, Takebe Y, Ibaragi S, Okui T, Kurio N, Takada H, Obata K, Pang P, Iwamoto M, Nagatsuka H, Sasaki A (2016) The role of sonic hedgehog signaling in osteoclastogenesis and jaw bone destruction. PLoS One 11(3):e151731
Guo Y, Yuan Y, Wu L, Ho TV, Jing J, Sugii H, Li J, Han X, Feng J, Guo C, Chai Y (2018) Bmp-ihh-mediated interplay between mesenchymal stem cells and osteoclasts supports calvarial bone homeostasis and repair. Bone Res 6:30
Heller E, Hurchla M, Xiang J, Su X, Chen S, Schneider J, Joeng K, Vidal M, Goldberg L, Deng H, Hornick M, Prior J, Piwnica-Worms D, Long F, Cagan R, Weilbaecher K (2012) Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res 72(4):897–907
Zhang L, Yang Y, Liao Z, Liu Q, Lei X, Li M, Saijilafu Z, Zhang D, Hong M, Zhu B, Li H, Yang JC (2020) Genetic and pharmacological activation of hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the jnk/c-fos-nfatc1 cascade. Theranostics 10(15):6638–6660
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell 130(5):811–823
Gao B, Lin X, Jing H, Fan J, Ji C, Jie Q, Zheng C, Wang D, Xu X, Hu Y, Lu W, Luo Z, Yang L (2018) Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell 17(3):e12741
Lu W, Gao B, Fan J, Cheng P, Hu Y, Jie Q, Luo Z, Yang L (2019) Mesenchymal progenitors derived from different locations in long bones display diverse characteristics. Stem Cells Int 2019:5037578
Qu C, Kunkalla K, Vaghefi A, Frederiksen JK, Liu Y, Chapman JR, Blonska M, Bernal-Mizrachi L, Alderuccio JP, Lossos IS, Landgraf R, Vega F (2018) Smoothened stabilizes and protects traf6 from degradation: a novel non-canonical role of smoothened with implications in lymphoma biology. Cancer Lett 436:149–158
Negishi-Koga T, Takayanagi H (2012) Bone cell communication factors and semaphorins. Bonekey Rep 1:183
Pederson L, Ruan M, Westendorf J, Khosla S, Oursler M (2008) Regulation of bone formation by osteoclasts involves wnt/bmp signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105(52):20764–20769
Henriksen K, Karsdal MA, Martin T (2014) Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int 94(1):88–97
Veis DJ, O’Brien CA (2022) Osteoclasts, master sculptors of bone. Annu Rev Pathol 18:257–281
Zaidi M, Troen B, Moonga B, Abe E (2001) Cathepsin k, osteoclastic resorption, and osteoporosis therapy. J Bone Miner Res 16(10):1747–1749
Lu J, Wang M, Wang Z, Fu Z, Lu A, Zhang G (2018) Advances in the discovery of cathepsin k inhibitors on bone resorption. J Enzyme Inhib Med Chem 33(1):890–904
Zheng Z, Zhang X, Huang B, Liu J, Wei X, Shan Z, Wu H, Feng Z, Chen Y, Fan S, Zhao F, Chen J (2021) Site-1 protease controls osteoclastogenesis by mediating lc3 transcription. Cell Death Differ 28(6):2001–2018
Lee SY, Park KH, Lee G, Kim SJ, Song WH, Kwon SH, Koh JT, Huh YH, Ryu JH (2021) Hypoxia-inducible factor-2alpha mediates senescence-associated intrinsic mechanisms of age-related bone loss. Exp Mol Med 53(4):591–604
Yang W, Wang J, Moore D, Liang H, Dooner M, Wu Q, Terek R, Chen Q, Ehrlich M, Quesenberry P, Neel B (2013) Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 499(7459):491–495
Debnath S, Yallowitz A, Mccormick J, Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang Y, Eiseman M, Shim J, Hameed M, Healey J, Bostrom M, Landau D, Greenblatt M (2018) Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562(7725):133–139
Feng H, Xing W, Han Y, Sun J, Kong M, Gao B, Yang Y, Yin Z, Chen X, Zhao Y, Bi Q, Zou W (2020) Tendon-derived cathepsin k-expressing progenitor cells activate hedgehog signaling to drive heterotopic ossification. J Clin Investig 130(12):6354–6365
Kohara Y, Haraguchi R, Kitazawa R, Imai Y, Kitazawa S (2020) Hedgehog inhibitors suppress osteoclastogenesis in in vitro cultures, and deletion of smo in macrophage/osteoclast lineage prevents age-related bone loss. Int J Mol Sci 21(8):2745
Sakunrangsit N, Pholtaisong J, Sucharitakul J, Wanna-Udom S, Prombutara P, Pisitkun P, Leelahavanichkul A, Aporntewan C, Greenblatt MB, Lotinun S (2021) Identification of candidate regulators of mandibular bone loss in fcgammariib(–/–) mice. Sci Rep 11(1):18726
Li J, Cui Y, Xu J, Wang Q, Yang X, Li Y, Zhang X, Qiu M, Zhang Z, Zhang Z (2017) Suppressor of fused restraint of hedgehog activity level is critical for osteogenic proliferation and differentiation during calvarial bone development. J Biol Chem 292(38):15814–15825
Miao D, Liu H, Plut P, Niu M, Huo R, Goltzman D, Henderson J (2004) Impaired endochondral bone development and osteopenia in gli2-deficient mice. Exp Cell Res 294(1):210–222
Johnson R, Nguyen M, Padalecki S, Grubbs B, Merkel A, Oyajobi B, Matrisian L, Mundy G, Sterling J (2011) Tgf-beta promotion of gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical hedgehog signaling. Cancer Res 71(3):822–831
Cannonier S, Gonzales C, Ely K, Guelcher S, Sterling J (2016) Hedgehog and tgfbeta signaling converge on gli2 to control bony invasion and bone destruction in oral squamous cell carcinoma. Oncotarget 7(46):76062–76075
Qu C, Liu Y, Kunkalla K, Singh R, Blonska M, Lin X, Agarwal N, Vega F (2013) Trimeric g protein-carma1 axis links smoothened, the hedgehog receptor transducer, to nf-kappab activation in diffuse large b-cell lymphoma. Blood 121(23):4718–4728
Matsuhashi Y, Tasaka T, Uehara E, Fujimoto M, Fujita M, Tamura T, Honda T, Kuwajima M, Shimoura Y, Mano S, Nagai M, Ishida T (2004) Diffuse large b-cell lymphoma presenting with hypercalcemia and multiple osteolysis. Leuk Lymphoma 45(2):397–400
Najafova Z, Liu P, Wegwitz F, Ahmad M, Tamon L, Kosinsky R, Xie W, Johnsen S, Tuckermann J (2021) Rnf40 exerts stage-dependent functions in differentiating osteoblasts and is essential for bone cell crosstalk. Cell Death Differ 28(2):700–714
Ohba S, Kawaguchi H, Kugimiya F, Ogasawara T, Kawamura N, Saito T, Ikeda T, Fujii K, Miyajima T, Kuramochi A, Miyashita T, Oda H, Nakamura K, Takato T, Chung U (2008) Patched1 haploinsufficiency increases adult bone mass and modulates gli3 repressor activity. Dev Cell 14(5):689–699
Safadi F, Xu J, Smock S, Kanaan R, Selim A, Odgren P, Marks SJ, Owen T, Popoff S (2003) Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol 196(1):51–62
Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M (2000) Effects of ctgf/hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol 184(2):197–206
Marinkovic M, Dai Q, Gonzalez AO, Tran ON, Block TJ, Harris SE, Salmon AB, Yeh CK, Dean DD, Chen XD (2022) Matrix-bound cyr61/ccn1 is required to retain the properties of the bone marrow mesenchymal stem cell niche but is depleted with aging. Matrix Biol 111:108–132
Acknowledgements
We thank Prof. Baojie Li for providing Smoflox/flox mice. We also thank Prof. Minghao Zheng for a kind gift of bovine bone slices.
Funding
This research was financially supported by the National Natural Science Foundation of China (81772377, 81871743, 81902202, 82130070), Innovation Team Projects—Innovation Capability Support Program of Shaanxi Province (2020TD-036), and Clinical Medical Research Center Projects—Innovation Capability Support Program of Shaanxi Province (2020LCZX-03). Key Research and Development Program of Shaanxi (2021SF-024).
Author information
Authors and Affiliations
Contributions
WL, QJ and LY designed the study and wrote and prepared the manuscript and figures. HZ and HW performed experiments on CKO mice. WL and CZ assisted experiments including western blot and qPCR in vitro. PC and CZ provided support about histomorphology and microCT analysis. SM and TH contributed to the operation and treatment. JF and HL performed mouse feeding and genotyping. YH and XH provided statistical analysis. LJ drew a schematic picture. ZL and JX revised this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, W., Zheng, C., Zhang, H. et al. Hedgehog signaling regulates bone homeostasis through orchestrating osteoclast differentiation and osteoclast–osteoblast coupling. Cell. Mol. Life Sci. 80, 171 (2023). https://doi.org/10.1007/s00018-023-04821-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04821-9