Abstract
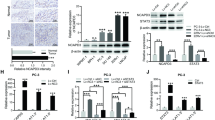
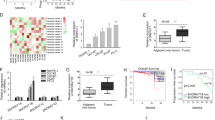
It has been reported that heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) is highly expressed in prostate cancer (PCa) and associated with poor prognosis of patients with PCa. Nevertheless, the specific mechanism underlying HNRNPA2B1 functions in PCa remains not clear. In our study, we proved that HNRNPA2B1 promoted the progression of PCa through in vitro and in vivo experiments. Further, we found that HNRNPA2B1 induced the maturation of miR-25-3p/miR-93-5p by recognizing primary miR-25/93 (pri-miR-25/93) through N6-methyladenosine (m6A)-dependent manner. In addition, both miR-93-5p and miR-25-3p were proven as tumor promoters in PCa. Interestingly, by mass spectrometry analysis and mechanical experiments, we found that casein kinase 1 delta (CSNK1D) could mediate the phosphorylation of HNRNPA2B1 to enhance its stability. Moreover, we further proved that miR-93-5p targeted BMP and activin membrane-bound inhibitor (BAMBI) mRNA to reduce its expression, thereby activating transforming growth factor β (TGF-β) pathway. At the same time, miR-25-3p targeted forkhead box O3 (FOXO3) to inactivate FOXO pathway. These results collectively indicated that CSNK1D stabilized HNRNPA2B1 facilitates the processing of miR-25-3p/miR-93-5p to regulate TGF-β and FOXO pathways, resulting in PCa progression. Our findings supported that HNRNPA2B1 might be a promising target for PCa treatment.









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
Teo MY, Rathkopf DE, Kantoff P (2019) Treatment of advanced prostate cancer. Annu Rev Med 70:479–499
Dai D, Wang H, Zhu L, Jin H, Wang X (2018) N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis 9:124
Wang X, Lu Z, Gomez A et al (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505:117–120
Wu B, Su S, Patil DP et al (2018) Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9:420
Alarcón CR, Goodarzi H, Lee H et al (2015) HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 162:1299–1308
Gupta A, Yadav S, Pt A et al (2020) The HNRNPA2B1-MST1R-Akt axis contributes to epithelial-to-mesenchymal transition in head and neck cancer. Lab Invest 100:1589–1601
Yang Y, Wei Q, Tang Y et al (2020) Loss of hnRNPA2B1 inhibits malignant capability and promotes apoptosis via down-regulating Lin28B expression in ovarian cancer. Cancer Lett 475:43–52
Hu Y, Sun Z, Deng J et al (2017) Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biol 39:1010428317694318
Stockley J, Villasevil ME, Nixon C et al (2014) The RNA-binding protein hnRNPA2 regulates β-catenin protein expression and is overexpressed in prostate cancer. RNA Biol 11:755–765
Ji G, Huang C, He S et al (2020) Comprehensive analysis of m6A regulators prognostic value in prostate cancer. Aging (Albany NY) 12:14863–14884
Tutar L, Özgür A, Tutar Y (2018) Involvement of miRNAs and pseudogenes in cancer. Methods Mol Biol 1699:45–66
Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF (2015) N6-methyladenosine marks primary microRNAs for processing. Nature 519:482–485
Lu TX, Rothenberg ME (2018) MicroRNA. J Allergy Clin Immunol 141:1202–1207
Fabris L, Ceder Y, Chinnaiyan AM et al (2016) The potential of MicroRNAs as prostate cancer biomarkers. Eur Urol 70:312–322
Zi Z (2019) Molecular engineering of the TGF-β signaling pathway. J Mol Biol 431:2644–2654
Thakur N, Hamidi A, Song J, et al. (2020) Smad7 enhances TGF-β-induced transcription of c-jun and HDAC6 promoting invasion of prostate cancer cells. iScience 23:101470.
Link W (2019) Introduction to FOXO biology. Methods Mol Biol 1890:1–9
Yan Y, Huang H (2019) Interplay among PI3K/AKT, PTEN/FOXO and AR signaling in prostate cancer. Adv Exp Med Biol 1210:319–331
Xu J, Chen Q, Tian K et al (2020) m6A methyltransferase METTL3 maintains colon cancer tumorigenicity by suppressing SOCS2 to promote cell proliferation. Oncol Rep 44:973–986
Chava S, Reynolds CP, Pathania AS et al (2020) miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol 14:180–196
Petri BJ, Piell KM, South Whitt GC et al (2021) HNRNPA2B1 regulates tamoxifen- and fulvestrant-sensitivity and hallmarks of endocrine resistance in breast cancer cells. Cancer Lett 518:152–168
Dai S, Zhang J, Huang S et al (2017) HNRNPA2B1 regulates the epithelial-mesenchymal transition in pancreatic cancer cells through the ERK/snail signalling pathway. Cancer Cell Int 17:12
Gao LB, Zhu XL, Shi JX et al (2021) HnRNPA2B1 promotes the proliferation of breast cancer MCF-7 cells via the STAT3 pathway. J Cell Biochem 122:472–484
Liu ZX, Li LM, Sun HL, Liu SM (2018) Link between m6A modification and cancers. Front Bioeng Biotechnol 6:89
Coker H, Wei G, Brockdorff N (2019) m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim Biophys Acta Gene Regul Mech 1862:310–318
Yang G, Sun Z, Zhang N (2020) Reshaping the role of m6A modification in cancer transcriptome: a review. Cancer Cell Int 20:353
Ma XX, Cao ZG, Zhao SL (2020) m6A methyltransferase METTL3 promotes the progression of prostate cancer via m6A-modified LEF1. Eur Rev Med Pharmacol Sci 24:3565–3571
Li J, Xie H, Ying Y et al (2020) YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer 19:152
Guo H, Wang B, Xu K et al (2020) m(6)A reader HNRNPA2B1 promotes esophageal cancer progression via up-regulation of ACLY and ACC1. Front Oncol 10:553045
Chen XY, Zhang J, Zhu JS (2019) The role of m(6)A RNA methylation in human cancer. Mol Cancer 18:103
Klinge CM, Piell KM, Tooley CS, Rouchka EC (2019) HNRNPA2/B1 is upregulated in endocrine-resistant LCC9 breast cancer cells and alters the miRNA transcriptome when overexpressed in MCF-7 cells. Sci Rep 9:9430
Eng GWL, Edison VDM (2017) Site-specific phosphorylation of casein kinase 1 δ (CK1δ) regulates its activity towards the circadian regulator PER2. PLoS One 12:e0177834
Rao HC, Wu ZK, Wei SD et al (2020) MiR-25-3p serves as an oncogenic MicroRNA by downregulating the expression of merlin in osteosarcoma. Cancer Manag Res 12:8989–9001
Peng G, Yang C, Liu Y, Shen C (2019) miR-25-3p promotes glioma cell proliferation and migration by targeting FBXW7 and DKK3. Exp Ther Med 18:769–778
Wan W, Wan W, Long Y et al (2019) MiR-25-3p promotes malignant phenotypes of retinoblastoma by regulating PTEN/Akt pathway. Biomed Pharmacother 118:109111
Zhang L, Tong Z, Sun Z, et al. (2020) MiR-25–3p targets PTEN to regulate the migration, invasion, and apoptosis of esophageal cancer cells via the PI3K/AKT pathway. Biosci Rep 40
Kong Z, Deng T, Zhang M et al (2018) β-arrestin1-medieated inhibition of FOXO3a contributes to prostate cancer cell growth in vitro and in vivo. Cancer Sci 109:1834–1842
Shan Z, Li Y, Yu S et al (2019) CTCF regulates the FoxO signaling pathway to affect the progression of prostate cancer. J Cell Mol Med 23:3130–3139
Li L, Zhao J, Huang S et al (2018) MiR-93-5p promotes gastric cancer-cell progression via inactivation of the Hippo signaling pathway. Gene 641:240–247
Ma DH, Li BS, Liu JJ et al (2017) miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett 408:23–32
Cao Y, Xia F, Wang P, Gao M (2018) MicroRNA-93-5p promotes the progression of human retinoblastoma by regulating the PTEN/PI3K/AKT signaling pathway. Mol Med Rep 18:5807–5814
Li Y, Zhang B, Xiang L et al (2020) TGF-β causes Docetaxel resistance in Prostate Cancer via the induction of Bcl-2 by acetylated KLF5 and Protein Stabilization. Theranostics 10:7656–7670
Funding
This work was supported by grants from Young Talents Program of Jiangsu Cancer Hospital (No. 2017YQL-04), and The Research Project of Jiangsu Cancer Hospital (ZM202015).
Author information
Authors and Affiliations
Contributions
XL and CQ designed the study and reviewed the data. FQ and WYS performed experiments and drafted the manuscript. XYW and YFC performed bioinformatics analyses. All the authors have read, revised, and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethics approval
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University.
Consent to participate
All patients participating in the study voluntarily donated samples for clinical research and signed a consent form before surgery.
Consent to publish
The authors affirm the research consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, F., Shen, W., Wei, X. et al. CSNK1D-mediated phosphorylation of HNRNPA2B1 induces miR-25-3p/miR-93-5p maturation to promote prostate cancer cell proliferation and migration through m6A-dependent manner. Cell. Mol. Life Sci. 80, 156 (2023). https://doi.org/10.1007/s00018-023-04798-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04798-5