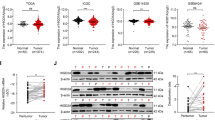
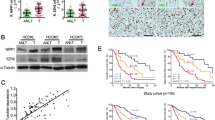
Abstract
Background
Hypoxia-inducible factors (HIFs) are the most essential endogenous transcription factors in the hypoxic microenvironment and regulate multiple genes involved in the proliferation, migration, invasion, and EMT of hepatocellular carcinoma (HCC) cells. However, the regulatory mechanism of HIFs in driving HCC progression remains poorly understood.
Methods
Gain- and loss-of-function experiments were carried out to investigate the role of TMEM237 in vitro and in vivo. The molecular mechanisms involved in HIF-1α-induced TMEM237 expression and TMEM237-mediated enhancement of HCC progression were confirmed by luciferase reporter, ChIP, IP-MS and Co-IP assays.
Results
TMEM237 was identified as a novel hypoxia-responsive gene in HCC. HIF-1α directly bound to the promoter of TMEM237 to transactivate its expression. The overexpression of TMEM237 was frequently detected in HCC and associated with poor clinical outcomes in patients. TMEM237 facilitated the proliferation, migration, invasion, and EMT of HCC cells and promoted tumor growth and metastasis in mice. TMEM237 interacted with NPHP1 and strengthened the interaction between NPHP1 and Pyk2 to trigger the phosphorylation of Pyk2 and ERK1/2, thereby contributing to HCC progression. The TMEM237/NPHP1 axis mediates hypoxia-induced activation of the Pyk2/ERK1/2 pathway in HCC cells.
Conclusions
Our study demonstrated that HIF-1α-activated TMEM237 interacted with NPHP1 to activate the Pyk2/ERK pathway, thereby promoting HCC progression.







Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included either in this article or in the supplementary information files.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HIFs:
-
Hypoxia-inducible factors
- PHD:
-
Prolyl hydroxylase
- HREs:
-
Hypoxia-responsive elements
- TMEM237:
-
Transmembrane protein 237
- JSRDs:
-
Joubert syndrome-related disorders
- EMT:
-
Epithelial–mesenchymal transition
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7:6
Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ et al (2018) Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 69:1284–1293
Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14:430–439
Prabhakar NR, Semenza GL (2015) Oxygen sensing and homeostasis. Physiology (Bethesda) 30:340–348
Xiong XX, Qiu XY, Hu DX, Chen XQ (2017) Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma. Mol Pharmacol 92:246–255
Yao B, Li Y, Chen T, Niu Y, Wang Y, Yang Y, Wei X, Liu Q, Tu K (2021) Hypoxia-induced cofilin 1 promotes hepatocellular carcinoma progression by regulating the PLD1/AKT pathway. Clin Transl Med 11:e366
Wang Y, Lyu Y, Tu K, Xu Q, Yang Y, Salman S, Le N, Lu H, Chen C, Zhu Y et al (2021) Histone citrullination by PADI4 is required for HIF-dependent transcriptional responses to hypoxia and tumor vascularization. Sci Adv 7:eabe3771
Sun L, Wang L, Chen T, Shi Y, Yao B, Liu Z, Wang Y, Li Q, Liu R, Niu Y et al (2020) LncRNA RUNX1-IT1 which is downregulated by hypoxia-driven histone deacetylase 3 represses proliferation and cancer stem-like properties in hepatocellular carcinoma cells. Cell Death Dis 11:95
Chen T, Liu R, Niu Y, Mo H, Wang H, Lu Y, Wang L, Sun L, Wang Y, Tu K, Liu Q (2021) HIF-1alpha-activated long non-coding RNA KDM4A-AS1 promotes hepatocellular carcinoma progression via the miR-411-5p/KPNA2/AKT pathway. Cell Death Dis 12:1152
Wang L, Sun L, Liu R, Mo H, Niu Y, Chen T, Wang Y, Han S, Tu K, Liu Q (2021) Long non-coding RNA MAPKAPK5-AS1/PLAGL2/HIF-1alpha signaling loop promotes hepatocellular carcinoma progression. J Exp Clin Cancer Res 40:72
Babcock JJ, Li M (2014) Deorphanizing the human transmembrane genome: a landscape of uncharacterized membrane proteins. Acta Pharmacol Sin 35:11–23
Marx S, Dal Maso T, Chen JW, Bury M, Wouters J, Michiels C, Le Calve B (2020) Transmembrane (TMEM) protein family members: poorly characterized even if essential for the metastatic process. Semin Cancer Biol 60:96–106
Schmit K, Michiels C (2018) TMEM proteins in cancer: a review. Front Pharmacol 9:1345
Shiraishi T, Ikeda K, Tsukada Y, Nishizawa Y, Sasaki T, Ito M, Kojima M, Ishii G, Tsumura R, Saijou S et al (2021) High expression of TMEM180, a novel tumour marker, is associated with poor survival in stage III colorectal cancer. BMC Cancer 21:302
Cheng Z, Guo J, Chen L, Luo N, Yang W, Qu X (2015) Overexpression of TMEM158 contributes to ovarian carcinogenesis. J Exp Clin Cancer Res 34:75
Zhang S, Dai H, Li W, Wang R, Wu H, Shen M, Hu Y, Xie L, Xing Y (2021) TMEM116 is required for lung cancer cell motility and metastasis through PDK1 signaling pathway. Cell Death Dis 12:1086
Zhang TM, Liao L, Yang SY, Huang MY, Zhang YL, Deng L, Hu SY, Yang F, Zhang FL, Shao ZM, Li DQ (2023) TOLLIP-mediated autophagic degradation pathway links the VCP-TMEM63A-DERL1 signaling axis to triple-negative breast cancer progression. Autophagy 19:805–821
Duan J, Qian Y, Fu X, Chen M, Liu K, Liu H, Yang J, Liu C, Chang Y (2021) TMEM106C contributes to the malignant characteristics and poor prognosis of hepatocellular carcinoma. Aging (Albany NY) 13:5585–5606
Rao J, Wu X, Zhou X, Deng R, Ma Y (2020) TMEM205 is an independent prognostic factor and is associated with immune cell infiltrates in hepatocellular carcinoma. Front Genet 11:575776
Zuniga FI, Craft CM (2010) Deciphering the structure and function of Als2cr4 in the mouse retina. Investig Ophthalmol Vis Sci 51:4407–4415
Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN et al (2011) TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet 89:713–730
Chen T, Sun L, Yao B, Wang L, Wang Y, Niu Y, Liu R, Mo H, Liu Z, Tu K, Liu Q (2020) MicroRNA-875-5p inhibits tumor growth and metastasis of hepatocellular carcinoma by targeting eukaryotic translation initiation factor 3 subunit a. Oncol Rep 44:2067–2079
Gao S, Chen T, Li L, Liu X, Liu Y, Zhao J, Lu Q, Zeng Z, Xu Q, Huang D, Tu K (2020) Hypoxia-inducible ubiquitin specific peptidase 13 contributes to tumor growth and metastasis via enhancing the toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-kappaB pathway in hepatocellular carcinoma. Front Cell Dev Biol 8:587389
Tang Z, Kang B, Li C, Chen T, Zhang Z (2019) GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 47:W556–W560
Benzing T, Gerke P, Hopker K, Hildebrandt F, Kim E, Walz G (2001) Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci USA 98:9784–9789
Revuelta-Lopez E, Castellano J, Roura S, Galvez-Monton C, Nasarre L, Benitez S, Bayes-Genis A, Badimon L, Llorente-Cortes V (2013) Hypoxia induces metalloproteinase-9 activation and human vascular smooth muscle cell migration through low-density lipoprotein receptor-related protein 1-mediated Pyk2 phosphorylation. Arterioscler Thromb Vasc Biol 33:2877–2887
Chen J, Wang Y, Zhang W, Zhao D, Zhang L, Fan J, Li J, Zhan Q (2020) Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct Target Ther 5:139
Schito L, Semenza GL (2016) Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2:758–770
Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL (2018) Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med 50:134
Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang D, Li T, Wang CZ, Tan YX, Ding J et al (2016) ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. J Hepatol 65:314–324
Zhao Q, Zhang L, He Q, Chang H, Wang Z, Cao H, Zhou Y, Pan R, Chen Y (2023) Targeting TRMT5 suppresses hepatocellular carcinoma progression via inhibiting the HIF-1alpha pathways. J Zhejiang Univ Sci B 24:50–63
Lin W, Li S, Meng Y, Huang G, Liang S, Du J, Liu Q, Cheng B (2021) UDCA inhibits hypoxic hepatocellular carcinoma cell-induced angiogenesis through suppressing HIF-1alpha/VEGF/IL-8 intercellular signaling. Front Pharmacol 12:755394
Cheng W, Cheng Z, Weng L, Xing D, Zhang M (2021) Asparagus polysaccharide inhibits the hypoxia-induced migration, invasion and angiogenesis of hepatocellular carcinoma cells partly through regulating HIF1alpha/VEGF expression via MAPK and PI3K signaling pathway. J Cancer 12:3920–3929
Zheng Y, Huang C, Lu L, Yu K, Zhao J, Chen M, Liu L, Sun Q, Lin Z, Zheng J et al (2021) STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol 14:16
Fernandez-Palanca P, Payo-Serafin T, San-Miguel B, Mendez-Blanco C, Tunon MJ, Gonzalez-Gallego J, Mauriz JL (2022) Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol Sin https://doi.org/10.1038/s41401-022-01021-2
Lambacher NJ, Bruel AL, van Dam TJ, Szymanska K, Slaats GG, Kuhns S, McManus GJ, Kennedy JE, Gaff K, Wu KM et al (2016) TMEM107 recruits ciliopathy proteins to subdomains of the ciliary transition zone and causes Joubert syndrome. Nat Cell Biol 18:122–131
Sabui S, Subramanian VS, Pham Q, Said HM (2019) Identification of transmembrane protein 237 as a novel interactor with the intestinal riboflavin transporter-3 (RFVT-3): role in functionality and cell biology. Am J Physiol Cell Physiol 316:C805–C814
Hildebrandt F, Otto E (2000) Molecular genetics of nephronophthisis and medullary cystic kidney disease. J Am Soc Nephrol 11:1753–1761
Beitner-Johnson D, Ferguson T, Rust RT, Kobayashi S, Millhorn DE (2002) Calcium-dependent activation of Pyk2 by hypoxia. Cell Signal 14:133–137
Lu H, Chen I, Shimoda LA, Park Y, Zhang C, Tran L, Zhang H, Semenza GL (2017) Chemotherapy-induced Ca(2+) release stimulates breast cancer stem cell enrichment. Cell Rep 18:1946–1957
Funding
This study was supported by grants from the National Natural Science Foundation of China (82203759), Nature Science Basic Research Program of Shaanxi (2020JC-36), Key Research and Development Program of Shaanxi (2023-YBSF-149), Innovation Capacity Support Plan in Shaanxi Province of China (2023KJXX-107) and Fundamental Research Funds for the Central Universities (xzy012022095).
Author information
Authors and Affiliations
Contributions
KT and QL conceived and designed the experiments; TC, LW, CC, RL, NZ, RL, and YN performed the experiments; TC, HL and ZX analyzed the data; HL and ZX contributed reagents/materials/analysis tools; TC and KT wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University and with the 1964 Helsinki declaration and its later amendments. All written informed consent to participate in the study was obtained from HCC patients for samples to be collected from them.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2023_4767_MOESM4_ESM.tif
Supplementary Figure 1 The correlations of TMEM237 expression with HIF-1α and TMEM237 CNV in HCC. (A) TCGA data analysis indicated that TMEM237 CNV was positively correlated with TMEM237 expression in HCC tissues. (B) TCGA data analysis demonstrated that HIF-1α was positively correlated with TMEM237 expression in HCC tissues. (C) TCGA data analysis revealed that HIF-1α was not associated with TMEM237 CNV in HCC tissues. (D) The proportion of TCGA-LIHC samples with TEME237 copy gain or loss. (E) A positive correlation between HIF-1α and TMEM237 expression was observed in HCC samples with TMEM237 copy gains. (F) A positive correlation between HIF-1α and TMEM237 expression was detected in HCC samples without TMEM237 copy gains (TIF 373 KB)
18_2023_4767_MOESM5_ESM.tif
Supplementary Figure 2 The expression and clinical significance of TMEM237 in HCC. (A) The difference in TMEM237 expression between HCC and nontumor tissues from the GEO database (GSE45436). (B and C) TCGA data were analyzed to determine TMEM237 expression in HCC tissues with different tumor grades and stages. (D) TCGA data analysis confirmed that TMEM237 CNV was not associated with the prognosis of HCC patients (TIF 153 KB)
18_2023_4767_MOESM6_ESM.tif
Supplementary Figure 3 The differentially expressed genes in HCCLM3 cells with or without TMEM237 knockdown. HCCLM3 cells that were transfected with shNC or shTMEM237 were analyzed by RNA-seq. (A) Volcano map of differentially expressed genes in HCCLM3 cells with or without TMEM237 knockdown. (B) Heatmap of differentially expressed genes in HCCLM3 cells with or without TMEM237 knockdown (TIF 263 KB)
18_2023_4767_MOESM8_ESM.tif
Supplementary Figure 5 TMEM237 promotes the proliferation and invasion of HCC cells. (A) Colony formation, (B) EdU, and (C) Transwell assays were conducted to evaluate the effects of TMEM237 overexpression and knockdown on the proliferation, migration and invasion of HCC cells. Scale bar: 50 μm for the EdU results and 200 μm for the transwell results (TIF 6157 KB)
18_2023_4767_MOESM11_ESM.tif
Supplementary Figure 8 The NPHP1/Pyk2/ERK1/2 pathway mediates the biological function of TMEM237 in HCC cells. (A) Colony formation, (B) EdU, and (C) transwell assays were employed to examine the proliferation, migration, and invasion of HCC cells in the indicated groups. Scale bar: 50 μm for the EdU results and 200 μm for the transwell results (TIF 8630 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, T., Wang, L., Chen, C. et al. HIF-1α-activated TMEM237 promotes hepatocellular carcinoma progression via the NPHP1/Pyk2/ERK pathway. Cell. Mol. Life Sci. 80, 120 (2023). https://doi.org/10.1007/s00018-023-04767-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04767-y