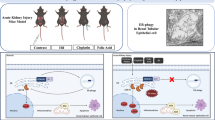
Abstract
Background
Cisplatin is an effective chemotherapeutic drug, but it may induce both acute and chronic kidney problems. The pathogenesis of chronic kidney disease (CKD) associated with cisplatin chemotherapy remains largely unclear.
Methods
Mice and renal tubular cells were subjected to repeated low-dose cisplatin (RLDC) treatment to induce CKD and related pathological changes. The roles of endoplasmic reticulum (ER) stress, PERK, and protein kinase C-δ (PKCδ) were determined using pharmacological inhibitors and genetic manipulation.
Results
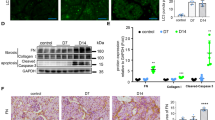
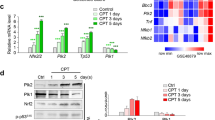
ER stress was induced by RLDC in kidney tubular cells in both in vivo and in vitro models. ER stress inhibitors given immediately after RLDC attenuated kidney dysfunction, tubular atrophy, kidney fibrosis, and inflammation in mice. In cultured renal proximal tubular cells, inhibitors of ER stress or its signaling kinase PERK also suppressed RLDC-induced fibrotic changes and the expression of inflammatory cytokines. Interestingly, RLDC-induced PKCδ activation, which was blocked by ER stress or PERK inhibitors, suggesting PKCδ may act downstream of PERK. Indeed, suppression of PKCδ with a kinase-dead PKCδ (PKCδ-KD) or Pkcδ-shRNA attenuated RLDC-induced fibrotic and inflammatory changes. Moreover, the expression of active PKCδ-catalytic fragment (PKCδ-CF) diminished the beneficial effects of PERK inhibitor in RLDC-treated cells. Co-immunoprecipitation assay further suggested PERK binding to PKCδ.
Conclusion
These results indicate that ER stress contributes to chronic kidney pathologies following cisplatin chemotherapy via the PERK–PKCδ pathway.







Similar content being viewed by others
Data availability
All data supporting the findings during this study are available in this manuscript and supplementary files.
Abbreviations
- CKD:
-
Chronic kidney disease
- RLDC:
-
Repeated low-dose cisplatin
- ER:
-
Endoplasmic reticulum
- PKCδ:
-
Protein kinase C-δ
- PKCδ-KD:
-
Kinase-dead PKCδ
- PKCδ-CF:
-
Active PKCδ-catalytic fragment
- AKI:
-
Acute kidney injury
- UPR:
-
Unfolded protein response
- NF-κB:
-
Nuclear factor-kappa B
- FN:
-
Fibronectin
- TUDCA:
-
Tauroursodeoxycholic acid
- 4-PBA:
-
4-Phenylbutyric acid
- HE:
-
Hematoxylin–eosin
- BUN:
-
Blood urea nitrogen
- GFR:
-
Glomerular filtration rate
- Mcp-1:
-
Monocyte chemoattractant protein-1
- Cxcl1:
-
C–X–C motif chemokine ligand 1
- W:
-
Week
- M:
-
Month
References
Cao W, Yuan Y, Liu X, Li Q, An X, Huang Z, Wu L, Zhang B, Zhang A, Xing C (2019) Adenosine kinase inhibition protects against cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 317(1):F107–F115. https://doi.org/10.1152/ajprenal.00385.2018
Cao X, Nie X, Xiong S, Cao L, Wu Z, Moore PK, Bian JS (2018) Renal protective effect of polysulfide in cisplatin-induced nephrotoxicity. Redox Biol 15:513–521. https://doi.org/10.1016/j.redox.2018.01.012
Deng B, Lin Y, Ma S, Zheng Y, Yang X, Li B, Yu W, Xu Q, Liu T, Hao C, He R, Ding F (2017) The leukotriene B4-leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney Int 92(1):89–100. https://doi.org/10.1016/j.kint.2017.01.009
Galgamuwa R, Hardy K, Dahlstrom JE, Blackburn AC, Wium E, Rooke M, Cappello JY, Tummala P, Patel HR, Chuah A, Tian L, McMorrow L, Board PG, Theodoratos A (2016) Dichloroacetate prevents cisplatin-induced nephrotoxicity without compromising cisplatin anticancer properties. J Am Soc Nephrol 27(11):3331–3344. https://doi.org/10.1681/ASN.2015070827
Gu X, Yang H, Sheng X, Ko YA, Qiu C, Park J, Huang S, Kember R, Judy RL, Park J, Damrauer SM, Nadkarni G, Loos RJF, My VTH, Chaudhary K, Bottinger EP, Paranjpe I, Saha A, Brown C, Akilesh S, Hung AM, Palmer M, Baras A, Overton JD, Reid J, Ritchie M, Rader DJ, Susztak K (2021) Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaz1458
Humanes B, Camano S, Lara JM, Sabbisetti V, Gonzalez-Nicolas MA, Bonventre JV, Tejedor A, Lazaro A (2017) Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol Dial Transplant 32(10):1645–1655. https://doi.org/10.1093/ndt/gfx005
Jung YJ, Park W, Kang KP, Kim W (2020) SIRT2 is involved in cisplatin-induced acute kidney injury through regulation of mitogen-activated protein kinase phosphatase-1. Nephrol Dial Transplant 35(7):1145–1156. https://doi.org/10.1093/ndt/gfaa042
Kumar G, Solanki MH, Xue X, Mintz R, Madankumar S, Chatterjee PK, Metz CN (2017) Magnesium improves cisplatin-mediated tumor killing while protecting against cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 313(2):F339–F350. https://doi.org/10.1152/ajprenal.00688.2016
Li Z, Xu K, Zhang N, Amador G, Wang Y, Zhao S, Li L, Qiu Y, Wang Z (2018) Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int 93(4):881–892. https://doi.org/10.1016/j.kint.2017.10.021
Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76(3):277–285. https://doi.org/10.1038/ki.2009.157
Oh CJ, Ha CM, Choi YK, Park S, Choe MS, Jeoung NH, Huh YH, Kim HJ, Kweon HS, Lee JM, Lee SJ, Jeon JH, Harris RA, Park KG, Lee IK (2017) Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int 91(4):880–895. https://doi.org/10.1016/j.kint.2016.10.011
Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, Wu M, Sparks MA, Privratsky JR, Herrera M, Gurley SB, Nedospasov SA, Crowley SD (2016) Competing actions of type 1 Angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27(8):2257–2264. https://doi.org/10.1681/ASN.2015060683
Zhang J, Zhao T, Wang C, Meng Q, Huo X, Wang C, Sun P, Sun H, Ma X, Wu J, Liu K (2021) Catalpol-induced AMPK activation alleviates cisplatin-induced nephrotoxicity through the mitochondrial-dependent pathway without compromising its anticancer properties. Oxid Med Cell Longev 2021:7467156. https://doi.org/10.1155/2021/7467156
Zhou J, An C, Jin X, Hu Z, Safirstein RL, Wang Y (2020) TAK1 deficiency attenuates cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 318(1):F209–F215. https://doi.org/10.1152/ajprenal.00516.2019
Burns CV, Edwin SB, Szpunar S, Forman J (2021) Cisplatin-induced nephrotoxicity in an outpatient setting. Pharmacotherapy 41(2):184–190. https://doi.org/10.1002/phar.2500
Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD (2016) Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol 11(7):1173–1179. https://doi.org/10.2215/CJN.08070715
Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD (2009) Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. Eur J Cancer 45(18):3213–3219. https://doi.org/10.1016/j.ejca.2009.06.032
Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, Jiang Y, Cutter GR, Boddu R, George JF, Agarwal A (2018) Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol 315(4):F1107–F1118. https://doi.org/10.1152/ajprenal.00179.2018
Katagiri D, Hamasaki Y, Doi K, Negishi K, Sugaya T, Nangaku M, Noiri E (2016) Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int 89(2):374–385. https://doi.org/10.1038/ki.2015.327
Li S, Lin Q, Shao X, Mou S, Gu L, Wang L, Zhang Z, Shen J, Zhou Y, Qi C, Jin H, Pang H, Ni Z (2019) NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp Cell Res 383(1):111488. https://doi.org/10.1016/j.yexcr.2019.07.001
Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ (2018) Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Renal Physiol 315(1):F161–F172. https://doi.org/10.1152/ajprenal.00636.2017
Arga M, Oguz A, Pinarli FG, Karadeniz C, Citak EC, Emeksiz HC, Duran EA, Soylemezoglu O (2015) Risk factors for cisplatin-induced long-term nephrotoxicity in pediatric cancer survivors. Pediatr Int 57(3):406–413. https://doi.org/10.1111/ped.12542
Green DM, Wang M, Krasin M, Srivastava D, Onder S, Jay DW, Ness KK, Greene W, Lanctot JQ, Shelton KC, Zhu L, Mulrooney DA, Ehrhardt MJ, Davidoff AM, Robison LL, Hudson MM (2021) Kidney function after treatment for childhood cancer: a report from the St. Jude Lifetime Cohort Study. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020060849
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110(10):1389–1398. https://doi.org/10.1172/JCI16886
Marciniak SJ, Chambers JE, Ron D (2021) Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-021-00320-3
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7(9):880–885. https://doi.org/10.1038/sj.embor.7400779
Charbonneau ME, O’Riordan MXD (2020) Reducing stress PERKs up anti-tumor immunity. Immunity 52(4):575–577. https://doi.org/10.1016/j.immuni.2020.03.012
Cunha DA, Cito M, Carlsson PO, Vanderwinden JM, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M (2016) Thrombospondin 1 protects pancreatic beta-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ 23(12):1995–2006. https://doi.org/10.1038/cdd.2016.89
Lei Z, Yue Y, Stone S, Wu S, Lin W (2020) NF-kappaB activation accounts for the cytoprotective effects of PERK activation on oligodendrocytes during EAE. J Neurosci 40(33):6444–6456. https://doi.org/10.1523/JNEUROSCI.1156-20.2020
Li W, Zhou X, Cai J, Zhao F, Cao T, Ning L, Luo C, Xiao X, Liu S (2021) Recombinant Treponema pallidum protein Tp0768 promotes proinflammatory cytokine secretion of macrophages through ER stress and ROS/NF-kappaB pathway. Appl Microbiol Biotechnol 105(1):353–366. https://doi.org/10.1007/s00253-020-11018-8
Qiao Q, Sun C, Han C, Han N, Zhang M, Li G (2017) Endoplasmic reticulum stress pathway PERK-eIF2alpha confers radioresistance in oropharyngeal carcinoma by activating NF-kappaB. Cancer Sci 108(7):1421–1431. https://doi.org/10.1111/cas.13260
Fan Y, Xiao W, Lee K, Salem F, Wen J, He L, Zhang J, Fei Y, Cheng D, Bao H, Liu Y, Lin F, Jiang G, Guo Z, Wang N, He JC (2017) Inhibition of reticulon-1A-mediated endoplasmic reticulum stress in early AKI attenuates renal fibrosis development. J Am Soc Nephrol 28(7):2007–2021. https://doi.org/10.1681/asn.2016091001
Inagi R, Ishimoto Y, Nangaku M (2014) Proteostasis in endoplasmic reticulum–new mechanisms in kidney disease. Nat Rev Nephrol 10(7):369–378. https://doi.org/10.1038/nrneph.2014.67
Shu S, Wang H, Zhu J, Liu Z, Yang D, Wu W, Cai J, Chen A, Tang C, Dong Z (2021) Reciprocal regulation between ER stress and autophagy in renal tubular fibrosis and apoptosis. Cell Death Dis 12(11):1016. https://doi.org/10.1038/s41419-021-04274-7
Shu S, Zhu J, Liu Z, Tang C, Cai J, Dong Z (2018) Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 37:269–280. https://doi.org/10.1016/j.ebiom.2018.10.006
Wei XM, Jiang S, Li SS, Sun YS, Wang SH, Liu WC, Wang Z, Wang YP, Zhang R, Li W (2021) Endoplasmic reticulum stress-activated PERK-eIF2alpha-ATF4 signaling pathway is involved in the ameliorative effects of ginseng polysaccharides against cisplatin-induced nephrotoxicity in mice. ACS Omega 6(13):8958–8966. https://doi.org/10.1021/acsomega.0c06339
Qvit N, Mochly-Rosen D (2014) The many hats of protein kinase C delta: one enzyme with many functions. Biochem Soc Trans 42(6):1529–1533. https://doi.org/10.1042/BST20140189
Greene MW, Ruhoff MS, Burrington CM, Garofalo RS, Orena SJ (2010) TNFalpha activation of PKCdelta, mediated by NFkappaB and ER stress, cross-talks with the insulin signaling cascade. Cell Signal 22(2):274–284. https://doi.org/10.1016/j.cellsig.2009.09.029
Steinberg SF (2008) Structural basis of protein kinase C isoform function. Physiol Rev 88(4):1341–1378. https://doi.org/10.1152/physrev.00034.2007
Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z (2011) Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121(7):2709–2722. https://doi.org/10.1172/JCI45586
Zhang D, Pan J, Xiang X, Liu Y, Dong G, Livingston MJ, Chen JK, Yin XM, Dong Z (2017) Protein kinase cdelta suppresses autophagy to induce kidney cell apoptosis in cisplatin nephrotoxicity. J Am Soc Nephrol 28(4):1131–1144. https://doi.org/10.1681/ASN.2016030337
Zheng M, Cai J, Liu Z, Shu S, Wang Y, Tang C, Dong Z (2019) Nicotinamide reduces renal interstitial fibrosis by suppressing tubular injury and inflammation. J Cell Mol Med 23(6):3995–4004. https://doi.org/10.1111/jcmm.14285
Fu Y, Cai J, Li F, Liu Z, Shu S, Wang Y, Liu Y, Tang C, Dong Z (2019) Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am J Physiol Renal Physiol 317(6):F1582–F1592. https://doi.org/10.1152/ajprenal.00385.2019
Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12(6):976–998. https://doi.org/10.1080/15548627.2016.1166317
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7(12):684–696. https://doi.org/10.1038/nrneph.2011.149
Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C, Acute Dialysis Quality Initiative Consensus XWG (2016) Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27(2):371–379. https://doi.org/10.1681/ASN.2015030261
Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, Kumar R (2013) Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 73(6):1993–2002. https://doi.org/10.1158/0008-5472.CAN-12-3109
Ferre S, Deng Y, Huen SC, Lu CY, Scherer PE, Igarashi P, Moe OW (2019) Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney Int 96(6):1359–1373. https://doi.org/10.1016/j.kint.2019.06.023
Liu H, Baliga R (2005) Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16(7):1985–1992. https://doi.org/10.1681/ASN.2004090768
Carlisle RE, Mohammed-Ali Z, Lu C, Yousof T, Tat V, Nademi S, MacDonald ME, Austin RC, Dickhout JG (2021) TDAG51 induces renal interstitial fibrosis through modulation of TGF-beta receptor 1 in chronic kidney disease. Cell Death Dis 12(10):921. https://doi.org/10.1038/s41419-021-04197-3
Chen YT, Jhao PY, Hung CT, Wu YF, Lin SJ, Chiang WC, Lin SL, Yang KC (2021) Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-beta signaling in kidney fibroblasts. J Clin Invest. https://doi.org/10.1172/JCI143645
Lee SJ, Kim SJ, Lee HS, Kwon OS (2019) PKCdelta mediates NF-kappaB inflammatory response and downregulates SIRT1 expression in liver fibrosis. Int J Mol Sci. https://doi.org/10.3390/ijms20184607
Chichger H, Vang A, O’Connell KA, Zhang P, Mende U, Harrington EO, Choudhary G (2015) PKC delta and betaII regulate angiotensin II-mediated fibrosis through p38: a mechanism of RV fibrosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308(8):L827–L836. https://doi.org/10.1152/ajplung.00184.2014
Wang J, Sun L, Nie Y, Duan S, Zhang T, Wang W, Ye RD, Hou S, Qian F (2020) Protein kinase C delta (PKCdelta) attenuates bleomycin induced pulmonary fibrosis via inhibiting NF-kappaB signaling pathway. Front Physiol 11:367. https://doi.org/10.3389/fphys.2020.00367
Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C, Group AXW (2016) Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27(3):687–697. https://doi.org/10.1681/ASN.2015030309
Canaud G, Brooks CR, Kishi S, Taguchi K, Nishimura K, Magassa S, Scott A, Hsiao LL, Ichimura T, Terzi F, Yang L, Bonventre JV (2019) Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aav4754
Ferenbach DA, Bonventre JV (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11(5):264–276. https://doi.org/10.1038/nrneph.2015.3
Tang C, Ma Z, Zhu J, Liu Z, Liu Y, Liu Y, Cai J, Dong Z (2019) P53 in kidney injury and repair: mechanism and therapeutic potentials. Pharmacol Ther 195:5–12. https://doi.org/10.1016/j.pharmthera.2018.10.013
Zheng Z, Li C, Shao G, Li J, Xu K, Zhao Z, Zhang Z, Liu J, Wu H (2021) Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis 12(8):754. https://doi.org/10.1038/s41419-021-04041-8
Njau F, Haller H (2021) Calcium dobesilate modulates PKCdelta-NADPH oxidase- MAPK-NF-kappaB signaling pathway to reduce CD14, TLR4, and MMP9 expression during monocyte-to-macrophage differentiation: potential therapeutic implications for atherosclerosis. Antioxidants (Basel). https://doi.org/10.3390/antiox10111798
Ren J, Wang Q, Morgan S, Si Y, Ravichander A, Dou C, Kent K, Liu B (2014) Protein kinase C-δ (PKCδ) regulates proinflammatory chemokine expression through cytosolic interaction with the NF-κB subunit p65 in vascular smooth muscle cells. J Biol Chem 289(13):9013–9026. https://doi.org/10.1074/jbc.M113.515957
Al Fayi M, Otifi H, Alshyarba M, Dera AA, Rajagopalan P (2020) Thymoquinone and curcumin combination protects cisplatin-induced kidney injury, nephrotoxicity by attenuating NFkappaB, KIM-1 and ameliorating Nrf2/HO-1 signalling. J Drug Target 28(9):913–922. https://doi.org/10.1080/1061186X.2020.1722136
Fujikura T, Yasuda H, Iwakura T, Tsuji T, Anders HJ (2019) MDM2 inhibitor ameliorates cisplatin-induced nephropathy via NFkappaBeta signal inhibition. Pharmacol Res Perspect 7(1):e00450. https://doi.org/10.1002/prp2.450
Yu C, Dong H, Wang Q, Bai J, Li YN, Zhao JJ, Li JZ (2021) Danshensu attenuates cisplatin-induced nephrotoxicity through activation of Nrf2 pathway and inhibition of NF-kappaB. Biomed Pharmacother 142:111995. https://doi.org/10.1016/j.biopha.2021.111995
Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding G, Huang S, Zhang A, Jia Z (2018) Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-kappaB and improving mitochondrial function. EBioMedicine 36:266–280. https://doi.org/10.1016/j.ebiom.2018.09.031
Zhang L, Gu Y, Li H, Cao H, Liu B, Zhang H, Shao F (2018) Daphnetin protects against cisplatin-induced nephrotoxicity by inhibiting inflammatory and oxidative response. Int Immunopharmacol 65:402–407. https://doi.org/10.1016/j.intimp.2018.10.018
Docherty MH, O’Sullivan ED, Bonventre JV, Ferenbach DA (2019) Cellular senescence in the kidney. J Am Soc Nephrol 30(5):726–736. https://doi.org/10.1681/ASN.2018121251
Acknowledgements
The authors would like to thank Huadong Medicine Co., Ltd (Hangzhou, China) for providing technical support of transcutaneous measurement of GFR in this study.
Funding
This study was financially supported by the National Key R&D Program of China [2020YFC2005004] and the National Natural Science Foundation of China [81720108008, 81870474].
Author information
Authors and Affiliations
Contributions
ZD, SS, and CT designed the study. SS and HW did most of the experiments. ZD, SS, HW, and CT performed data analysis. All authors contributed to the preparation, writing, and final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared no competing interests.
Ethics approval
All animal experiments were performed following the protocol approved by the Animal Ethics Committee of The Second Xiangya Hospital of Central South University with the Approval number 2020076.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4480_MOESM1_ESM.tif
Supplementary Fig. 1 Endoplasmic reticulum (ER) stress is activated in mouse kidneys post-repeated low-dose cisplatin (RLDC) treatment. C57BL/6 male mice were subjected to four weekly injections of cisplatin (8 mg/kg) to collect blood and kidney tissues 1 week (1W) or 1 month (1M) later. FITC-Sinistrin was injected via tail vein before sacrifice to measure glomerular filtration rate (GFR). (a) Diagram of cisplatin treatment. (b) Quantitative analysis of GFR. (c) Concentration of serum creatinine. (d) Concentration of blood urea nitrogen (BUN). (e) Representative HE staining images. Bar= 100 μm. (f) Pathological tubular atrophy score. (g, h) Representative images of Masson trichrome staining and quantitative analysis. Bar= 100 μm. (i, j) Immunoblot analysis of p-PERK, PERK, p-eIF2α, eIF2α, and GAPDH. For quantification, the protein was analyzed through densitometry and then normalized with GAPDH. (k, l) Representative immunohistochemical staining images and quantification of p-PERK expression. Bar= 100 μm. N =6 mice. *p < 0.05; **p < 0.01; ***p < 0.001
18_2022_4480_MOESM2_ESM.tif
Supplementary Fig. 2 4-PBA and TUDCA inhibit ER stress post-RLDC treatment in kidney tubules. C57BL/6 male mice were subjected to four weekly injections of cisplatin (8 mg/kg). 4-PBA, TUDCA, or saline was given daily after the last injection of cisplatin for 1 week. Kidney tissues were collected 1 month after the last injection of cisplatin. (a–d) Immunoblot analysis of p-PERK, PERK, p-eIF2α, eIF2α, and GAPDH. For quantification, the protein was analyzed through densitometry and then normalized with GAPDH. (e, f) Representative immunohistochemical staining images and quantification of p-PERK expression. Bar= 100 μm. N =6 mice. ***p < 0.001
18_2022_4480_MOESM3_ESM.tif
Supplementary Fig. 3 ER stress is induced by RLDC treatment in BUMPT cells. BUMPT cells were incubated with different concentrations of cisplatin for 7 hours daily for 4 days. (a) Representative images of phase contrast. Bar= 100 μm. (b-e) Immunoblot analysis of FN, vimentin, p-PERK, PERK, p-eIF2α, eIF2α, and GAPDH. For quantification, the protein was analyzed through densitometry and then normalized with GAPDH. n =4. ***p < 0.001 vs. control
18_2022_4480_MOESM4_ESM.tif
Supplementary Fig. 4 PKCδ knockdown by Pkcδ-shRNA in BUMPT cells. BUMPT cells were transfected with or without Pkcδ-shRNA. (a–b) Immunoblot analysis of PKCδ and GAPDH. For quantification, the protein was analyzed through densitometry and then normalized with GAPDH. n =4. ***p < 0.001
Rights and permissions
About this article
Cite this article
Shu, S., Wang, H., Zhu, J. et al. Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK–PKCδ pathway. Cell. Mol. Life Sci. 79, 452 (2022). https://doi.org/10.1007/s00018-022-04480-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04480-2