Abstract
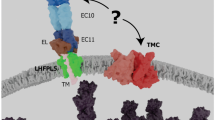
Sound signals are acquired and digitized in the cochlea by the hair cells that further transmit the coded information to the central auditory pathways. Any defect in hair cell function may induce problems in the auditory system and hearing-based brain function. In the past 2 decades, our understanding of auditory transduction has been substantially deepened because of advances in molecular, structural, and functional studies. Results from these experiments can be perfectly embedded in the previously established profile from anatomical, histological, genetic, and biophysical research. This review aims to summarize the progress on the molecular and cellular mechanisms of the mechano-electrical transduction (MET) channel in the cochlear hair cells, which is involved in the acquisition of sound frequency and intensity—the two major parameters of an acoustic cue. We also discuss recent studies on TMC1, the molecule likely to form the MET channel pore.



Similar content being viewed by others
References
Kandel ER et al (2012) Principles of neural science. McGraw-Hill Education / Medical; 5th edn (October 26, 2012), p 1760
Robles L, Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81(3):1305–1352
Fettiplace R, Kim KX (2014) The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev 94(3):951–986
Pietsch M et al (2017) Spiral form of the Human Cochlea results from spatial constraints. Sci Rep 7(1):7500
Von Bekesy G (1947) The variation of phase along the basilar membrane with sinusoidal vibrations. J Acoust Soc Am 19(3):452–460
Hubbard A (1993) A traveling-wave amplifier model of the cochlea. Science 259(5091):68–71
Lee HY et al (2015) Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc Natl Acad Sci U S A 112(10):3128–3133
Nankali A et al (2020) A role for tectorial membrane mechanics in activating the cochlear amplifier. Sci Rep 10(1):17620
Sellon JB et al (2015) Longitudinal spread of mechanical excitation through tectorial membrane traveling waves. Proc Natl Acad Sci U S A 112(42):12968–12973
Zheng J et al (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405(6783):149–155
Liberman MC et al (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419(6904):300–304
Kaltenbach JA, Falzarano PR (1994) Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. J Comp Neurol 340(1):87–97
Kaltenbach JA, Falzarano PR, Simpson TH (1994) Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J Comp Neurol 350(2):187–198
Soons JA et al (2015) Cytoarchitecture of the mouse organ of corti from base to apex, determined using in situ two-photon imaging. J Assoc Res Otolaryngol 16(1):47–66
Duvall AJ 3rd, Flock A, Wersall J (1966) The ultrastructure of the sensory hairs and associated organelles of the cochlear inner hair cell, with reference to directional sensitivity. J Cell Biol 29(3):497–505
Engstrom H, Engstrom B (1978) Structure of the hairs on cochlear sensory cells. Hear Res 1(1):49–66
Hudspeth AJ (2014) Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci 15(9):600–614
Fettiplace R, Hackney CM (2006) The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7(1):19–29
Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576(Pt 1):11–21
Zdebik AA, Wangemann P, Jentsch TJ (2009) Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 24:307–316
Gill SS, Salt AN (1997) Quantitative differences in endolymphatic calcium and endocochlear potential between pigmented and albino guinea pigs. Hear Res 113(1–2):191–197
Conlee JW, Bennett ML (1993) Turn-specific differences in the endocochlear potential between albino and pigmented guinea pigs. Hear Res 65(1–2):141–150
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281(5733):675–677
Kazmierczak P et al (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449(7158):87–91
Siemens J et al (2004) Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428(6986):950–955
Sollner C et al (2004) Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428(6986):955–959
Elledge HM et al (2010) Structure of the N terminus of cadherin 23 reveals a new adhesion mechanism for a subset of cadherin superfamily members. Proc Natl Acad Sci U S A 107(23):10708–10712
Sotomayor M et al (2010) Structural determinants of cadherin-23 function in hearing and deafness. Neuron 66(1):85–100
Sotomayor M et al (2012) Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492(7427):128–132
Beurg M et al (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12(5):553–558
Dionne G et al (2018) Mechanotransduction by PCDH15 relies on a novel cis -dimeric architecture. Neuron 99(3):480
Ge J et al (2018) Structure of mouse protocadherin 15 of the stereocilia tip link in complex with LHFPL5. Elife. https://doi.org/10.7554/eLife.387707
Bartsch TF et al (2019) Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing. Proc Natl Acad Sci U S A 116(22):11048–11056
Ahmed ZM et al (2006) The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26(26):7022–7034
Webb SW et al (2011) Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development 138(8):1607–1617
Maeda R et al (2017) Functional analysis of the transmembrane and cytoplasmic domains of Pcdh15a in Zebrafish hair cells. J Neurosci 37(12):3231–3245
Alagramam KN et al (2001) The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27(1):99–102
Alagramam KN et al (2001) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10(16):1709–1718
Bolz H et al (2001) Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27(1):108–112
Bork JM et al (2001) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68(1):26–37
Di Palma F et al (2001) Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet 27(1):103–107
Kurima K et al (2002) Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30(3):277–284
Kurima K et al (2003) Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis☆. Genomics 82(3):300–308
Vreugde S et al (2002) Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet 30(3):257–258
de Heer AM et al (2011) Progressive sensorineural hearing loss and normal vestibular function in a Dutch DFNB7/11 family with a novel mutation in TMC1. Audiol Neurootol 16(2):93–105
Kitajiri SI et al (2007) Identities, frequencies and origins of TMC1 mutations causing DFNB7/B11 deafness in Pakistan. Clin Genet 72(6):546–550
Tlili A et al (2008) TMC1 but not TMC2 is responsible for autosomal recessive nonsyndromic hearing impairment in Tunisian families. Audiol Neurootol 13(4):213–218
Marcotti W et al (2006) Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol 574(Pt 3):677–698
Beurg M et al (2019) A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc Natl Acad Sci U S A 116(41):20743–20749
Yu X et al (2020) Deafness mutation D572N of TMC1 destabilizes TMC1 expression by disrupting LHFPL5 binding. Proc Natl Acad Sci U S A 117(47):29894–29903
Kawashima Y et al (2011) Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121(12):4796–4809
Asai Y et al (2018) Transgenic Tmc2 expression preserves inner ear hair cells and vestibular function in mice lacking Tmc1. Sci Rep 8(1):12124
Nakanishi H et al (2018) Tmc2 expression partially restores auditory function in a mouse model of DFNB7/B11 deafness caused by loss of Tmc1 function. Sci Rep 8(1):12125
Lelli A et al (2009) Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 101(6):2961–2973
Waguespack J et al (2007) Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27(50):13890–13902
Kurima K et al (2015) TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell Stereocilia. Cell Rep 12(10):1606–1617
Beurg M et al (2018) Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat Commun 9(1):2185
Li X et al (2019) Localization of TMC1 and LHFPL5 in auditory hair cells in neonatal and adult mice. FASEB J 33(6):6838–6851
Cunningham CL et al (2020) tmie defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron 107(1):126
Ricci AJ, Crawford AC, Fettiplace R (2003) Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40(5):983–990
Beurg M et al (2006) A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26(43):10992–11000
Beurg M, Kim KX, Fettiplace R (2014) Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 144(1):55–69
Kim KX, Fettiplace R (2013) Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol 141(1):141–148
Pan B et al (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79(3):504–515
Beurg M, Goldring AC, Fettiplace R (2015) The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J Gen Physiol 146(3):233–243
Ballesteros A, Fenollar-Ferrer C, Swartz KJ (2018) Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. Elife. https://doi.org/10.7554/eLife.38433
Pan B et al (2018) TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99(4):736-753.e6
Maeda R et al (2014) Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci U S A 111(35):12907–12912
Beurg M et al (2015) Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci U S A 112(5):1589–1594
Labay V et al (2010) Topology of transmembrane channel-like gene 1 protein. Biochemistry 49(39):8592–8598
Jia Y et al (2019) TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron. https://doi.org/10.1016/j.neuron.2019.10.017
Tlili A et al (2005) A novel autosomal recessive non-syndromic deafness locus, DFNB66, maps to chromosome 6p21.2–22.3 in a large Tunisian consanguineous family. Hum Hered 60(3):123–128
Kalay E et al (2006) Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum Mutat 27(7):633–639
Shabbir MI et al (2006) Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43(8):634–640
Longo-Guess CM et al (2005) A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102(22):7894–7899
Longo-Guess CM et al (2007) Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation. Mamm Genome 18(9):646–656
Xiong W et al (2012) TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151(6):1283–1295
Cosetti M et al (2008) Unique transgenic animal model for hereditary hearing loss. Ann Otol Rhinol Laryngol 117(11):827–833
Zhao B et al (2014) TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84(5):954–967
Mitchem KL et al (2002) Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet 11(16):1887–1898
Naz S et al (2002) Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet 71(3):632–636
Santos RL et al (2006) Novel sequence variants in the TMIE gene in families with autosomal recessive nonsyndromic hearing impairment. J Mol Med (Berl) 84(3):226–231
Cunningham CL et al (2017) The murine catecholamine methyltransferase mTOMT is essential for mechanotransduction by cochlear hair cells. Elife. https://doi.org/10.7554/eLife.24318
Erickson T et al (2017) Integration of Tmc1/2 into the mechanotransduction complex in zebrafish hair cells is regulated by Transmembrane O-methyltransferase (Tomt). Elife. https://doi.org/10.7554/eLife.28474
Giese APJ et al (2017) CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Commun 8(1):43
Michel V et al (2017) CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol Med 9(12):1711–1731
Wang Y et al (2017) Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Front Mol Neurosci 10:401
Blazejczyk M et al (2009) Biochemical characterization and expression analysis of a novel EF-hand Ca2+ binding protein calmyrin2 (Cib2) in brain indicates its function in NMDA receptor mediated Ca2+ signaling. Arch Biochem Biophys 487(1):66–78
Riazuddin S et al (2012) Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44(11):1265–1271
Jan A (2013) Mutations in CIB2 calcium and integrin-binding protein disrupt auditory hair cell calcium homeostasis in Usher syndrome type 1J and non-syndromic deafness DFNB48. Clin Genet 83(4):317–318
Booth KT et al (2017) Variants in CIB2 cause DFNB48 and not USH1J. Clin Genet 93(4):812–821
Ahmed ZM et al (2008) Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans. Nat Genet 40(11):1335–1340
Du X et al (2008) A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc Natl Acad Sci USA 105(38):14609–14614
Ross CJ et al (2009) Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 41(12):1345–1349
Maoiléidigh OD, Ricci AJ (2019) A bundle of mechanisms: inner-ear hair-cell mechanotransduction. Trends Neurosci 42(3):221–236
Marquis RE, Hudspeth AJ (1997) Effects of extracellular Ca2+ concentration on hair-bundle stiffness and gating-spring integrity in hair cells. Proc Natl Acad Sci U S A 94(22):11923–11928
Tinevez JY, Julicher F, Martin P (2007) Unifying the various incarnations of active hair-bundle motility by the vertebrate hair cell. Biophys J 93(11):4053–4067
Beurg M et al (2008) The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94(7):2639–2653
Hacohen N et al (1989) Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J Neurosci 9(11):3988–3997
Kennedy HJ et al (2003) Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6(8):832–836
Kimitsuki T, Ohmori H (1992) The effect of caged calcium release on the adaptation of the transduction current in chick hair cells. J Physiol 458:27–40
Ricci AJ, Fettiplace R (1997) The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 501(Pt 1):111–124
Ricci AJ, Wu YC, Fettiplace R (1998) The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18(20):8261–8277
Peng AW, Effertz T, Ricci AJ (2013) Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80(4):960–972
Corns LF et al (2014) Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci U S A 111(41):14918–14923
Caprara GA et al (2019) Hair bundle stimulation mode modifies manifestations of mechanotransduction adaptation. J Neurosci 39(46):9098–9106
Hirono M et al (2004) Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44(2):309–320
Zhao H et al (2012) Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci 32(13):4600–4609
Goodyear RJ et al (2003) A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci 23(27):9208–9219
Sekerkova G et al (2006) Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol Life Sci 63(19–20):2329–2341
Suh BC, Hille B (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37:175–195
Peng AW et al (2016) Adaptation independent modulation of auditory hair cell mechanotransduction channel open probability implicates a role for the lipid bilayer. J Neurosci 36(10):2945–2956
Effertz T et al (2017) Phosphoinositol-4,5-bisphosphate regulates auditory hair-cell mechanotransduction-channel pore properties and fast adaptation. J Neurosci 37(48):11632–11646
Caprara GA, Mecca AA, Peng AW (2020) Decades-old model of slow adaptation in sensory hair cells is not supported in mammals. Sci Adv 6(33):eabb4922
Ricci AJ et al (2005) The transduction channel filter in auditory hair cells. J Neurosci 25(34):7831–7839
Krey JF et al (2020) Mechanotransduction-Dependent Control of Stereocilia Dimensions and Row Identity in Inner Hair Cells. Curr Biol 30(3):442-454e7
Liu S et al (2019) TMC1 is an essential component of a leak channel that modulates tonotopy and excitability of auditory hair cells in mice. Elife. https://doi.org/10.7554/eLife.47441
Rodriguez L et al (2012) Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc Natl Acad Sci U S A 109(35):14013–14018
Gianoli F, Risler T, Kozlov AS (2017) Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proc Natl Acad Sci U S A 114(51):E11010
Meyer J et al (1998) Evidence for opening of hair-cell transducer channels after tip-link loss. J Neurosci 18(17):6748–6756
Yue X et al (2019) Distinct functions of TMC channels: a comparative overview. Cell Mol Life Sci 76(21):4221–4232
Zhang L et al (2015) TMC-1 attenuates C elegans development and sexual behaviour in a chemically defined food environment. Nat Commun 6:6345
Wang X et al (2016) TMC-1 mediates alkaline sensation in C. elegans through nociceptive neurons. Neuron 91(1):146–154
Yue X et al (2018) TMC proteins modulate egg laying and membrane excitability through a background leak conductance in C. elegans. Neuron 97:1–15
Tang Y-Q et al (2020) Ankyrin is an intracellular tether for TMC mechanotransduction channels. Neuron 107(4):759
Guo Y et al (2016) Transmembrane channel-like (tmc) gene regulates Drosophila larval locomotion. Proc Natl Acad Sci U S A 113(26):7243–7248
Zhang YV et al (2016) The basis of food texture sensation in Drosophila. Neuron 91(4):863–877
He L et al (2019) Direction selectivity in Drosophila proprioceptors requires the mechanosensory channel Tmc. Curr Biol 29(6):945-956e3
Vaadia RD et al (2019) Characterization of proprioceptive system dynamics in behaving drosophila larvae using high-speed volumetric microscopy. Curr Biol 29(6):935–944
Pickles JO, Comis SD, Osborne MP (1984) Cross-links between Stereocilia in the Guinea-Pig organ of corti, and their possible relation to sensory transduction. Hear Res 15(2):103–112
Furness DN, Hackney CM (1985) Cross-Links between Stereocilia in the Guinea-Pig Cochlea. Hear Res 18(2):177–188
Xiao B (2020) Levering mechanically activated piezo channels for potential pharmacological intervention. Annu Rev Pharmacol Toxicol 60:195–218
Marcotti W et al (2003) Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol 548(Pt 2):383–400
Funding
This work was supported by the National Natural Science Foundation of China (31522025, 31571080, 81873703, and 31861163003), Beijing Municipal Science and Technology Commission (Z181100001518001), and a startup fund from the Tsinghua-Peking Center for Life Sciences. W.X. is a CIBR cooperative investigator (2020-NKX-XM-04) funded by the Open Collaborative Research Program of Chinese Institute for Brain Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Wang, S., Zou, L. et al. Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity. Cell. Mol. Life Sci. 78, 5083–5094 (2021). https://doi.org/10.1007/s00018-021-03840-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03840-8