Abstract
Background
Lung ischemia/reperfusion injury (LIRI) is a common occurrence in clinical practice and represents a significant complication following pulmonary transplantation and various diseases. At the core of pulmonary ischemia/reperfusion injury lies sterile inflammation, where the innate immune response plays a pivotal role. This review aims to investigate recent advancements in comprehending the role of innate immunity in LIRI.
Methods
A computer-based online search was performed using the PubMed database and Web of Science database for published articles concerning lung ischemia/reperfusion injury, cell death, damage-associated molecular pattern molecules (DAMPs), innate immune cells, innate immunity, inflammation.
Results
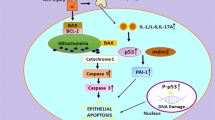
During the process of lung ischemia/reperfusion, cellular injury even death can occur. When cells are injured or undergo cell death, endogenous ligands known as DAMPs are released. These molecules can be recognized and bound by pattern recognition receptors (PRRs), leading to the recruitment and activation of innate immune cells. Subsequently, a cascade of inflammatory responses is triggered, ultimately exacerbating pulmonary injury. These steps are complex and interrelated rather than being in a linear relationship. In recent years, significant progress has been made in understanding the immunological mechanisms of LIRI, involving novel types of cell death, the ability of receptors other than PRRs to recognize DAMPs, and a more detailed mechanism of action of innate immune cells in ischemia/reperfusion injury (IRI), laying the groundwork for the development of novel diagnostic and therapeutic approaches.
Conclusions
Various immune components of the innate immune system play critical roles in lung injury after ischemia/reperfusion. Preventing cell death and the release of DAMPs, interrupting DAMPs receptor interactions, disrupting intracellular inflammatory signaling pathways, and minimizing immune cell recruitment are essential for lung protection in LIRI.



Similar content being viewed by others
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527–34.
Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med. 1986;314(18):1140–5.
Young KA, Dilling DF. The future of lung transplantation. Chest. 2019;155(3):465–73.
Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading-A 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Transpl. 2017;36(10):1097–103.
Chen-Yoshikawa TF. Ischemia–reperfusion injury in lung transplantation. Cells. 2021;10(6):1.
de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia–reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511.
Chatterjee S, Browning EA, Hong N, et al. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol. 2012;302(1):H105-114.
Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2014;307(9):L668-680.
Talaie T, DiChiacchio L, Prasad NK, et al. Ischemia–reperfusion injury in the transplanted lung: a literature review. Transplantation direct. 2021;7(2):e652.
Fischer S, Maclean AA, Liu M, et al. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia–reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162(5):1932–9.
Stammberger U, Gaspert A, Hillinger S, et al. Apoptosis induced by ischemia and reperfusion in experimental lung transplantation. Ann Thorac Surg. 2000;69(5):1532–6.
Fischer S, Cassivi SD, Xavier AM, et al. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg. 2000;231(3):424–31.
Ng CS, Wan S, Yim AP. Pulmonary ischaemia-reperfusion injury: role of apoptosis. Eur Respir J. 2005;25(2):356–63.
Quadri SM, Segall L, de Perrot M, et al. Caspase inhibition improves ischemia–reperfusion injury after lung transplantation. Am J Transpl Off J Am Soc Transplant Am Soc Transpl Surg. 2005;5(2):292–9.
Hashimoto K, Besla R, Zamel R, et al. Circulating cell death biomarkers may predict survival in human lung transplantation. Am J Respir Crit Care Med. 2016;194(1):97–105.
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149(7):1536–48.
Kim H, Zhao J, Zhang Q, et al. δV1-1 reduces pulmonary ischemia reperfusion-induced lung injury by inhibiting necrosis and mitochondrial localization of PKCδ and p53. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2016;16(1):83–98.
Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204(9):2089–102.
Capuzzimati M, Hough O, Liu M. Cell death and ischemia–reperfusion injury in lung transplantation. J Heart Lung Transpl. 2022;41(8):1003–13.
Wang X, O’Brien ME, Yu J, et al. Prolonged cold ischemia induces necroptotic cell death in ischemia–reperfusion injury and contributes to primary graft dysfunction after lung transplantation. Am J Respir Cell Mol Biol. 2019;61(2):244–56.
Kim H, Zamel R, Bai XH, et al. Ischemia–reperfusion induces death receptor-independent necroptosis via calpain-STAT3 activation in a lung transplant setting. Am J Physiol Lung Cell Mol Physiol. 2018;315(4):L595-l608.
Li W, Terada Y, Tyurina YY, et al. Necroptosis triggers spatially restricted neutrophil-mediated vascular damage during lung ischemia reperfusion injury. Proc Natl Acad Sci USA. 2022;119(10):e2111537119.
Wang L, Chen B, Xiong X, Chen S, Jin L, Zhu M. Necrostatin-1 synergizes the pan caspase inhibitor to attenuate lung injury induced by ischemia reperfusion in rats. Mediators Inflamm. 2020;2020:7059304.
Ueda S, Chen-Yoshikawa TF, Tanaka S, et al. Protective effect of necrosulfonamide on rat pulmonary ischemia–reperfusion injury via inhibition of necroptosis. J Thorac Cardiovasc Surg. 2022;163(2):e113–22.
Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21.
Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–27.
Dong L, Liang F, Lou Z, et al. Necrostatin-1 alleviates lung ischemia–reperfusion injury via inhibiting necroptosis and apoptosis of lung epithelial cells. Cells. 2022;11(19):1.
Matsui Y, Kanou T, Matsui T, et al. Protective effect of calpain inhibition during cold ischemia on ischemia–reperfusion injury after lung transplantation. Transplantation. 2023;2023:1.
Zhou P, Guo H, Li Y, et al. Monocytes promote pyroptosis of endothelial cells during lung ischemia–reperfusion via IL-1R/NF-κB/NLRP3 signaling. Life Sci. 2021;276:119402.
Noda K, Tane S, Haam SJ, et al. Targeting circulating leukocytes and pyroptosis during ex vivo lung perfusion improves lung preservation. Transplantation. 2017;101(12):2841–9.
Zhou P, Song NC, Zheng ZK, Li YQ, Li JS. MMP2 and MMP9 contribute to lung ischemia–reperfusion injury via promoting pyroptosis in mice. BMC Pulm Med. 2022;22(1):230.
Fei L, Jingyuan X, Fangte L, et al. Preconditioning with rHMGB1 ameliorates lung ischemia–reperfusion injury by inhibiting alveolar macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. J Transl Med. 2020;18(1):301.
Zheng P, Kang J, Xing E, Zheng B, Wang X, Zhou H. Lung inflation with hydrogen during the cold ischemia phase alleviates lung ischemia–reperfusion injury by inhibiting pyroptosis in rats. Front Physiol. 2021;12:699344.
Wu S, Li Z, Ye M, et al. VX765, a specific caspase-1 inhibitor, alleviates lung ischemia reperfusion injury by suppressing endothelial pyroptosis and barrier dysfunction. Biomed Res Int. 2021;2021:4525988.
Jin T, Ai F, Zhou J, et al. Emodin alleviates lung ischemia–reperfusion injury by suppressing gasdermin D-mediated pyroptosis in rats. Clin Respir J. 2023;17(3):241–50.
Li Y, Yang M, Xie L, Zhang G, Xu J, Xu S. Sulforaphane alleviates postresuscitation lung pyroptosis possibly via activating the NRF2/HO-1 pathway. Shock (Augusta). 2023;60(3):427–33.
Cloer CM, Givens CS, Buie LK, et al. Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion. J Heart Lung Transpl. 2023;42(5):575–84.
Jiang T, Liu T, Deng X, et al. Adiponectin ameliorates lung ischemia–reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir Res. 2021;22(1):258.
Ryter SW, Choi AM. Autophagy in the lung. Proc Am Thorac Soc. 2010;7(1):13–21.
Chen X, Wu JX, You XJ, Zhu HW, Wei JL, Xu MY. Cold ischemia-induced autophagy in rat lung tissue. Mol Med Rep. 2015;11(4):2513–9.
Lin HQ, Dai SH, Liu WC, et al. Effects of prolonged cold-ischemia on autophagy in the graft lung in a rat orthotopic lung transplantation model. Life Sci. 2021;268:118820.
Zhang D, Li C, Zhou J, et al. Autophagy protects against ischemia/reperfusion-induced lung injury through alleviating blood-air barrier damage. J Heart Lung Transpl. 2015;34(5):746–55.
Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85.
Xu Y, Li X, Cheng Y, Yang M, Wang R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia–reperfusion. FASEB J Off Publ Feder Am Soc Exp Biol. 2020;34(12):16262–75.
Wang Y, Dong Z, Zhang Z, Wang Y, Yang K, Li X. Postconditioning with irisin attenuates lung ischemia/reperfusion injury by suppressing ferroptosis via induction of the Nrf2/HO-1 signal axis. Oxid Med Cell Longev. 2022;2022:9911167.
Frye CC, Bery AI, Kreisel D, Kulkarni HS. Sterile inflammation in thoracic transplantation. Cell Mol Life Sci CMLS. 2021;78(2):581–601.
Arancibia SA, Beltrán CJ, Aguirre IM, et al. Toll-like receptors are key participants in innate immune responses. Biol Res. 2007;40(2):97–112.
Shepherd HM, Gauthier JM, Li W, Krupnick AS, Gelman AE, Kreisel D. Innate immunity in lung transplantation. J Heart Lung Transpl. 2021;40(7):562–8.
Merry HE, Phelan P, Doak MR, Zhao M, Hwang B, Mulligan MS. Role of toll-like receptor-4 in lung ischemia–reperfusion injury. Ann Thorac Surg. 2015;99(4):1193–9.
Zanotti G, Casiraghi M, Abano JB, et al. Novel critical role of Toll-like receptor 4 in lung ischemia–reperfusion injury and edema. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L52-63.
Prakash A, Mesa KR, Wilhelmsen K, Xu F, Dodd-o JM, Hellman J. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology. 2012;117(4):822–35.
Almeida FM, Battochio AS, Napoli JP, et al. Creatine supply attenuates ischemia–reperfusion injury in lung transplantation in rats. Nutrients. 2020;12(9):1.
He Q, Zhao X, Bi S, Cao Y. Pretreatment with erythropoietin attenuates lung ischemia/reperfusion injury via toll-like receptor-4/nuclear factor-κB (TLR4/NF-κB) pathway. Med Sci Monit Int Med J Exp Clin Res. 2018;24:1251–7.
Wang Y, Lin D, Tan H, Gao Y, Ma J. Penehyclidine hydrochloride preconditioning provides pulmonary and systemic protection in a rat model of lung ischaemia reperfusion injury. Eur J Pharmacol. 2018;839:1–11.
Querrey M, Chiu S, Lecuona E, et al. CD11b suppresses TLR activation of nonclassical monocytes to reduce primary graft dysfunction after lung transplantation. J Clin Investig. 2022. https://doi.org/10.1172/JCI157262.
Fei L, Jifeng F, Tiantian W, Yi H, Linghui P. Glycyrrhizin ameliorate ischemia reperfusion lung injury through downregulate TLR2 signaling cascade in alveolar macrophages. Front Pharmacol. 2017;8:389.
Phelan P, Merry HE, Hwang B, Mulligan MS. Differential toll-like receptor activation in lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2015;149(6):1653–61.
Zhang XY, Chen C, Zhang YB, et al. Role of toll-like receptor 3 in lung ischemia–reperfusion injury. Shock (Augusta). 2016;46(4):405–11. https://doi.org/10.1186/s12931-016-0472-y.
Mallavia B, Liu F, Lefrançais E, et al. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am J Respir Cell Mol Biol. 2020;62(3):364–72. https://doi.org/10.1165/rcmb.2019-0140OC.
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. https://doi.org/10.1038/nm1315
Ramnath D, Powell EE, Scholz GM, Sweet MJ. The toll-like receptor 3 pathway in homeostasis, responses to injury and wound repair. Semin Cell Dev Biol. 2017;61:22–30. https://doi.org/10.1016/j.semcdb.2016.08.014
Xu Z, Sharma M, Gelman A, Hachem R, Mohanakumar T. Significant role for microRNA-21 affecting toll-like receptor pathway in primary graft dysfunction after human lung transplantation. J Heart Lung Transplant. 2017;36(3):331–339. https://doi.org/10.1016/j.healun.2016.08.028
Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112.
Sakaguchi M, Murata H, Yamamoto K, et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS ONE. 2011;6(8):e23132.
Lapar DJ, Hajzus VA, Zhao Y, et al. Acute hyperglycemic exacerbation of lung ischemia–reperfusion injury is mediated by receptor for advanced glycation end-products signaling. Am J Respir Cell Mol Biol. 2012;46(3):299–305.
Lee S, Piao C, Kim G, Kim JY, Choi E, Lee M. Production and application of HMGB1 derived recombinant RAGE-antagonist peptide for anti-inflammatory therapy in acute lung injury. Eur J Pharmaceut Sci Off J Eur Feder Pharmaceut Sci. 2018;114:275–84.
Sharma AK, LaPar DJ, Stone ML, Zhao Y, Kron IL, Laubach VE. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia–reperfusion injury. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2013;13(9):2255–67.
Liang F, Liu H, He X, et al. Role and regulatory mechanism of triggering receptor expressed on myeloid cells 2 in mice lung ischemia/reperfusion injury. Zhonghua wei zhong bing ji jiu yi xue. 2021;33(8):933–7.
Liu H, Zhang L, Liu Z, et al. Galectin-3 as TREM2 upstream factor contributes to lung ischemia–reperfusion injury by regulating macrophage polarization. iScience. 2023;26(9):107496.
Leroy V, Cai J, Tu Z, et al. Resolution of post-lung transplant ischemia–reperfusion injury is modulated via Resolvin D1-FPR2 and Maresin 1-LGR6 signaling. J Heart Lung Transpl. 2023;42(5):562–74.
Oda H, Tanaka S, Shinohara M, et al. Specialized proresolving lipid meditators agonistic to formyl peptide receptor type 2 attenuate ischemia–reperfusion injury in rat lung. Transplantation. 2022;106(6):1159–69.
Duan L, Hu GH, Li YJ, Zhang CL, Jiang M. P2X7 receptor is involved in lung injuries induced by ischemia–reperfusion in pulmonary arterial hypertension rats. Mol Immunol. 2018;101:409–18.
Demertzis S, Langer F, Graeter T, Dwenger A, Georg T, Schäfers HJ. Amelioration of lung reperfusion injury by L- and E-selectin blockade. Eur J Cardiothor Surg Off J Eur Assoc Cardiothor Surg. 1999;16(2):174–80.
Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci USA. 1997;94(2):757–61.
DeMeester SR, Molinari MA, Shiraishi T, et al. Attenuation of rat lung isograft reperfusion injury with a combination of anti-ICAM-1 and anti-beta2 integrin monoclonal antibodies. Transplantation. 1996;62(10):1477–85.
Su G, Hodnett M, Wu N, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2007;36(3):377–86.
Mallavia B, Liu F, Sheppard D, Looney MR. Inhibiting integrin αvβ5 reduces ischemia–reperfusion injury in an orthotopic lung transplant model in mice. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2016;16(4):1306–11.
Zhang D, Li C, Song Y, et al. Integrin αvβ5 inhibition protects against ischemia–reperfusion-induced lung injury in an autophagy-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L384-l394.
Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990;85(4):1090–8.
Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52.
Hu R, Chen ZF, Yan J, et al. Endoplasmic reticulum stress of neutrophils is required for ischemia/reperfusion-induced acute lung injury. J Immunol (Baltimore, Md: 1950). 2015;195(10):4802–9.
Kulkarni HS, Ramphal K, Ma L, et al. Local complement activation is associated with primary graft dysfunction after lung transplantation. JCI Insight. 2020;5(17):1.
Hsiao HM, Fernandez R, Tanaka S, et al. Spleen-derived classical monocytes mediate lung ischemia–reperfusion injury through IL-1β. J Clin Investig. 2018;128(7):2833–47.
Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York). 2010;330(6005):841–5.
Hettinger J, Richards DM, Hansson J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14(8):821–30.
Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10-19.
Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35.
Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–75.
Kurihara C, Lecuona E, Wu Q, et al. Crosstalk between nonclassical monocytes and alveolar macrophages mediates transplant ischemia–reperfusion injury through classical monocyte recruitment. JCI Insight. 2021;6(6):1.
Zheng Z, Chiu S, Akbarpour M, et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci Transl Med. 2017;9(394):1.
Kreisel D, Nava RG, Li W, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA. 2010;107(42):18073–8.
Liu Y, Li W, Luehmann HP, et al. Noninvasive imaging of CCR2(+) cells in ischemia–reperfusion injury after lung transplantation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2016;16(10):3016–23.
Schmidl C, Renner K, Peter K, et al. Transcription and enhancer profiling in human monocyte subsets. Blood. 2014;123(17):e90-99.
Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Investig. 2019;129(7):2619–28.
Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (New York). 2012;336(6077):86–90.
Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210(10):1977–92.
Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804.
Kopecky BJ, Frye C, Terada Y, Balsara KR, Kreisel D, Lavine KJ. Role of donor macrophages after heart and lung transplantation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2020;20(5):1225–35.
Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121(6):1069–75.
Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1018-1026.
Naidu BV, Woolley SM, Farivar AS, et al. Early tumor necrosis factor-alpha release from the pulmonary macrophage in lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2004;127(5):1502–8.
Johansson A, Lundborg M, Sköld CM, et al. Functional, morphological, and phenotypical differences between rat alveolar and interstitial macrophages. Am J Respir Cell Mol Biol. 1997;16(5):582–8.
Shi T, Denney L, An H, Ho LP, Zheng Y. Alveolar and lung interstitial macrophages: definitions, functions, and roles in lung fibrosis. J Leukoc Biol. 2021;110(1):107–14.
Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L105-113.
Merry HE, Phelan P, Doaks M, Zhao M, Mulligan MS. Functional roles of tumor necrosis factor-alpha and interleukin 1-Beta in hypoxia and reoxygenation. Ann Thorac Surg. 2015;99(4):1200–5.
Li J, Han Z, Zhu Z, Wei L. LncRNA H19 aggravates primary graft dysfunction after lung transplantation via KLF5-mediated activation of CCL28. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2023;23(10):1536–50.
McCourtie AS, Merry HE, Farivar AS, Goss CH, Mulligan MS. Alveolar macrophage secretory products augment the response of rat pulmonary artery endothelial cells to hypoxia and reoxygenation. Ann Thorac Surg. 2008;85(3):1056–60.
Spahn JH, Li W, Bribriesco AC, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol (Baltimore, Md: 1950). 2015;194(8):4039–48.
Stein-Streilein J. Invariant NKT cells as initiators, licensors, and facilitators of the adaptive immune response. J Exp Med. 2003;198(12):1779–83.
He X, Xiao J, Li Z, et al. Inhibition of PD-1 alters the SHP1/2-PI3K/Akt axis to decrease M1 polarization of alveolar macrophages in lung ischemia–reperfusion injury. Inflammation. 2023;46(2):639–54.
Adoumie R, Serrick C, Giaid A, Shennib H. Early cellular events in the lung allograft. Ann Thor Surg. 1992;54(6):1071–6 (Discussion 1076–1077).
Shepherd HM, Gauthier JM, Terada Y, et al. Updated views on neutrophil responses in ischemia–reperfusion injury. Transplantation. 2022;106(12):2314–24.
Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med (Cambridge). 2011;17(3–4):293–307.
DiStasi MR, Ley K. Opening the flood-gates: how neutrophil–endothelial interactions regulate permeability. Trends Immunol. 2009;30(11):547–56.
Scozzi D, Liao F, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophil extracellular traps in acute lung injury. Front Immunol. 2022;13:953195.
Jiang P, Jin Y, Sun M, et al. Extracellular histones aggravate inflammation in ARDS by promoting alveolar macrophage pyroptosis. Mol Immunol. 2021;135:53–61.
Karki P, Birukov KG, Birukova AA. Extracellular histones in lung dysfunction: a new biomarker and therapeutic target? Pulmonary Circ. 2020;10(4):2045894020965357.
Hsieh IN, Deluna X, White MR, Hartshorn KL. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J Leukoc Biol. 2021;109(4):763–75.
Zhang Y, Guan L, Yu J, et al. Pulmonary endothelial activation caused by extracellular histones contributes to neutrophil activation in acute respiratory distress syndrome. Respir Res. 2016;17(1):155.
Sayah DM, Mallavia B, Liu F, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191(4):455–63.
Li J, Wei L, Han Z, Chen Z, Zhang Q. Long non-coding RNA X-inactive specific transcript silencing ameliorates primary graft dysfunction following lung transplantation through microRNA-21-dependent mechanism. EBioMedicine. 2020;52:102600.
Wienkamp AK, Erpenbeck L, Rossaint J. Platelets in the NETworks interweaving inflammation and thrombosis. Front Immunol. 2022;13:953129.
Yang SH, Lee JP, Jang HR, et al. Sulfatide-reactive natural killer T cells abrogate ischemia–reperfusion injury. J Am Soc Nephrol. 2011;22(7):1305–14.
Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218(2):246–50.
Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-17 mediates lung ischemia–reperfusion injury. Am J Respir Crit Care Med. 2011;183(11):1539–49.
Sharma AK, LaPar DJ, Stone ML, et al. NOX2 activation of natural killer T cells is blocked by the adenosine A2A receptor to inhibit lung ischemia–reperfusion injury. Am J Respir Crit Care Med. 2016;193(9):988–99.
Sharma AK, Mulloy DP, Le LT, Laubach VE. NADPH oxidase mediates synergistic effects of IL-17 and TNF-α on CXCL1 expression by epithelial cells after lung ischemia–reperfusion. Am J Physiol Lung Cell Mol Physiol. 2014;306(1):L69-79.
Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Investig. 2007;117(8):2302–12.
Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. 2020;180(6):1280-1280.e1281.
Calabrese DR, Aminian E, Mallavia B, et al. Natural killer cells activated through NKG2D mediate lung ischemia–reperfusion injury. J Clin Invest. 2021;131(3):1.
Monticelli LA, Diamond JM, Saenz SA, et al. Lung innate lymphoid cell composition is altered in primary graft dysfunction. Am J Respir Crit Care Med. 2020;201(1):63–72.
Everaere L, Ait-Yahia S, Molendi-Coste O, et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol. 2016;138(5):1309-1318.e1311.
Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671-678.e674.
Silver JS, Kearley J, Copenhaver AM, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17(6):626–35.
Hams E, Armstrong ME, Barlow JL, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci USA. 2014;111(1):367–72.
Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–82.
de Perrot M, Young K, Imai Y, et al. Recipient T cells mediate reperfusion injury after lung transplantation in the rat. J Immunol (Baltimore, Md: 1950). 2003;171(10):4995–5002.
Yamamoto S, Nava RG, Zhu J, et al. Cutting edge: pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol (Baltimore, Md: 1950). 2012;189(9):4221–5.
Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–8.
Nakajima J, Ono M, Takeda M, Kawauchi M, Furuse A, Takizawa H. Role of costimulatory molecules on airway epithelial cells acting as alloantigen-presenting cells. Transpl Proc. 1997;29(4):2297–300.
Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia–reperfusion injury. J Thor Cardiovasc Surg. 2009;137(3):695–702 (Discussion 702).
Liu B, Ding F, Cao D, Liu J, Wang Y, Wu C. Pseudomonas aeruginosa outer membrane vesicles ameliorates lung ischemia–reperfusion injury by regulating the balance of regulatory T cells and Th17 cells through Tim-3 and TLR4/NF-κB pathway. Inflam Res Off J Eur Hist Res Soc (et al). 2021;70(8):891–902.
Chen Z, Chen Y, Zhou J, Li Y, Gong C, Wang X. Netrin-1 reduces lung ischemia–reperfusion injury by increasing the proportion of regulatory T cells. J Int Med Res. 2020;48(6):300060520926415.
Akimova T, Zhang T, Christensen LM, et al. Obesity-related IL-18 impairs T-regulatory cell function and promotes lung ischemia–reperfusion injury. Am J Respir Crit Care Med. 2021;204(9):1060–74.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82170021).
Author information
Authors and Affiliations
Contributions
HN put forward the idea of the paper and made revisions to the manuscript. QL summarized the relevant research progress and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Written informed consent for publication was obtained from all participants.
Ethics approval and consent to participate
Not applicable.
Additional information
Responsible Editor: Anatolii Kubyshkin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Nie, H. Advances in lung ischemia/reperfusion injury: unraveling the role of innate immunity. Inflamm. Res. 73, 393–405 (2024). https://doi.org/10.1007/s00011-023-01844-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01844-7