Abstract
Objective
Sepsis may often result in acute lung injury (ALI), with a high mortality and morbidity. Available evidence indicates that activation of NLRP3 inflammasome to induce macrophage inflammation plays a crucial role in the inflammation progression of ALI and lidocaine can attenuate inflammatory responses. We hypothesized that lidocaine may attenuate inflammatory response and sepsis-induced ALI by inhibiting potassium efflux-dependent NLRP3 activation.
Methods
C57BL/6N mice were randomized and divided into six groups (n = 6) receiving different treatments. Lung vascular permeability and histological changes in the lungs were evaluated by Evans blue dye, bronchoalveolar lavage analysis and hematoxylin and eosin staining. J774A.1 macrophages were divided into 12 groups receiving different treatments. The expression of both NLRP3 inflammasome activation-related protein and P2X7 in the macrophages was measured by immunofluorescence staining and Western blots. The whole cell currents were determined by a voltage-patch clamp technique.
Results
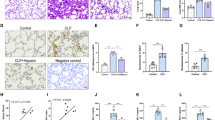
Challenge with LPS led to ALI in mice with an increased lung injury score (0.54 ± 0.09), which was significantly attenuated by lidocaine pretreatment (0.20 ± 0.08, P < 0.0001). Lidocaine pretreatment significantly decreased the NLRP3 activation and IL-1β release in the macrophages. Furthermore, lidocaine pretreatment down-regulated the expression of P2X7 receptors, inhibited LPS- and ATP-induced sodium (Na+) inward flow, and maintained the intracellular K+ level in the macrophages. In addition, activation of Na+ influx did not eliminate anti-inflammatory effect of lidocaine. The activation of NLRP3 could be suppressed by extracellular K+ level in a dose-dependent model. However, lidocaine pretreatment eliminated NLRP3 activation and IL-1β release induced by K+ efflux, and decreased outward K+ current and extracellular K+ level in the macrophages challenged by LPS/ATP.
Conclusions
Lidocaine pretreatment can attenuate the sepsis-induced ALI by an anti-inflammatory mechanism of inhibiting K+ efflux-dependent NLRP3 activation.








Similar content being viewed by others
Data availability
The datasets used during the present study are available from the corresponding author on reasonable request.
References
Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–22.
Habashi NM, Camporota L, Gatto LA, Nieman G. Functional pathophysiology of SARS-CoV-2-induced acute lung injury and clinical implications. J Appl Physiol. 1985;2021(130):877–91.
Goss CH, Brower RG, Hudson LD, Rubenfeld GD, Network A. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–11.
Zhou H, Fan EK, Fan J. Cell–cell interaction mechanisms in acute lung injury. Shock. 2021;55:167–76.
Fan EKY, Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res. 2018;19:50.
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53.
Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, et al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity. 2018;49(56–65): e4.
Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, Werdehausen R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123:335–49.
Kinoshita H, Ishikawa T, Hatano Y. Differential effects of lidocaine and mexiletine on relaxations to ATP-sensitive K+ channel openers in rat aortas. Anesthesiology. 1999;90:1165–70.
Yang X, Yang LX, Wu J, Guo ML, Zhang Y, Ma SG. Treatment of lidocaine on subacute thyroiditis via restraining inflammatory factor expression and inhibiting pyroptosis pathway. J Cell Biochem. 2019;120:10964–71.
Wang L, Wang M, Li S, Wu H, Shen Q, Zhang S, et al. Nebulized lidocaine ameliorates allergic airway inflammation via downregulation of TLR2. Mol Immunol. 2018;97:94–100.
Wang X, Wu FP, Huang YR, Li HD, Cao XY, You Y, et al. Matrine suppresses NLRP3 inflammasome activation via regulating PTPN2/JNK/SREBP2 pathway in sepsis. Phytomedicine. 2023;109: 154574.
Di A, Krupa B, Nelson DJ. Calcium-G protein interactions in the regulation of macrophage secretion. J Biol Chem. 2001;276:37124–32.
Zheng B, Yang H, Zhang J, Wang X, Sun H, Hu F, et al. Lidocaine alleviates sepsis-induced acute lung injury in mice by suppressing tissue factor and matrix metalloproteinase-2/9. Oxid Med Cell Longev. 2021;2021:3827501.
Jiang C, Liu Z, Hu R, Bo L, Minshall RD, Malik AB, Hu G. Inactivation of Rab11a GTPase in macrophages facilitates phagocytosis of apoptotic neutrophils. J Immunol. 2017;198:1660–72.
Su K, Bo L, Jiang C, Deng X, Zhao YY, et al. TLR4 is required for macrophage efferocytosis during resolution of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2021;321:L787–801.
Nasseri S, Gurusamy M, Jung B, Lee D, Khang G, Doods H, Wu D. Kinin B1 receptor antagonist BI113823 reduces acute lung injury. Crit Care Med. 2015;43:e499-507.
Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. Acute lung injury in animals Study G An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38.
Chen H, Ma N, Song X, Wei G, Zhang H, Liu J, et al. Protective effects of N-acetylcysteine on lipopolysaccharide-induced respiratory inflammation and oxidative stress. Antioxidants (Basel). 2022;11(5):879.
Xu J, Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48:331–44.
Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, et al. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–20.
Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–83.
Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–50.
Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20:270–84.
Caracas HC, Maciel JV, Martins PM, de Souza MM, Maia LC. The use of lidocaine as an anti-inflammatory substance: a systematic review. J Dent. 2009;37:93–7.
Lin S, Jin P, Shao C, Lu W, Xiang Q, Jiang Z, et al. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIF1alpha-induced glycolysis. Int Immunopharmacol. 2020;80: 106150.
Romera A, Cebollero M, Romero-Gomez B, Carricondo F, Zapatero S, Garcia-Aldao U, et al. Effect of intravenous lidocaine on inflammatory and apoptotic response of ischemia-reperfusion injury in pigs undergoing lung resection surgery. Biomed Res Int. 2021;2021:6630232.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Nova Z, Skovierova H, Calkovska A. Alveolar-capillary membrane-related pulmonary cells as a target in endotoxin-induced acute lung injury. Int J Mol Sci. 2019;20:831.
Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–68.
Feng Y, Li M, Yangzhong X, Zhang X, Zu A, Hou Y, et al. Pyroptosis in inflammation-related respiratory disease. J Physiol Biochem. 2022;78:721–37.
Di Virgilio F, Schmalzing G, Markwardt F. The Elusive P2X7 macropore. Trends Cell Biol. 2018;28:392–404.
Pelegrin P. P2X7 receptor and the NLRP3 inflammasome: partners in crime. Biochem Pharmacol. 2021;187: 114385.
Okura D, Horishita T, Ueno S, Yanagihara N, Sudo Y, Uezono Y, et al. Lidocaine preferentially inhibits the function of purinergic P2X7 receptors expressed in Xenopus oocytes. Anesth Analg. 2015;120:597–605.
Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14:121–37.
Acknowledgements
We thank Hua Wei, Ph.D. (Core Facilities Centre, Capital Medical University) for the help with the patch clamp.
Funding
This work was supported by the Funds for Beijing Municipal Natural Science Foundation (No. 7214221, to Kai Su), Capital Health Improvement and Research (No. 2018-4-1116, to Kai Su) and Bethune Charitable Foundation (No. SX2022009, to Kai Su).
Author information
Authors and Affiliations
Contributions
K. Su, X.T. Li, M. Jin and F. S. Xue conceived and designed the research; K. Su, X. T. Li and M. Jin performed the experiments; M. Jin and F. S. Xue analyzed the data; K. Su, F. X. Hong and F.S. Xue interpreted the results of experiments; K. Su and X.T. Li prepared the figures; K.Su, X. T. Li and F. S. Xue drafted the manuscript; M. Jin, F. X. Hong and F. S. Xue edited and revised the manuscript; K. Su, X.T. Li, F. X. Hong, M. Jin and F. S. Xue approved the final version of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2023_1810_MOESM1_ESM.tif
Effect of lidocaine pretreatment on IL-1β in the THP-1 macrophages challenged with the LPS. Data represent means ± SD. LPS, lipopolysaccharide; ATP, Adenosine triphosphate; Lido, lidocaine; p17, IL-1β p17. A and B: Expressions of IL-1β by western blotting. A, the independent western blots for IL-1β (17, 31 kDa) and GAPDH (37 kDa; loading control). B, quantification of western blotting data for IL-1β p17 as shown in A (n = 3. ★P < 0.05, §P < 0.0001). C: IL-1β level in the supernatant of cultured THP-1 macrophages (n = 6. §P < 0.0001). Supplementary file1 (TIF 1207 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, K., Li, XT., Hong, FX. et al. Lidocaine pretreatment attenuates inflammatory response and protects against sepsis-induced acute lung injury via inhibiting potassium efflux-dependent NLRP3 activation. Inflamm. Res. 72, 2221–2235 (2023). https://doi.org/10.1007/s00011-023-01810-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01810-3