Abstract
Background
Monocytes/macrophages play critical roles in inflammation and cardiac remodeling following myocardial infarction (MI). The cholinergic anti-inflammatory pathway (CAP) modulates local and systemic inflammatory responses by activating α7 nicotinic acetylcholine receptors (α7nAChR) in monocytes/macrophages. We investigated the effect of α7nAChR on MI-induced monocyte/macrophage recruitment and polarization and its contribution to cardiac remodeling and dysfunction.
Methods
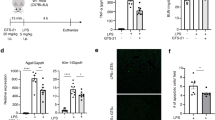
Adult male Sprague Dawley rats underwent coronary ligation and were intraperitoneally injected with the α7nAChR-selective agonist PNU282987 or the antagonist methyllycaconitine (MLA). RAW264.7 cells were stimulated with lipopolysaccharide (LPS) + interferon-gamma (IFN-γ) and treated with PNU282987, MLA, and S3I-201 (a STAT3 inhibitor). Cardiac function was evaluated by echocardiography. Masson’s trichrome and immunofluorescence were used to detect cardiac fibrosis, myocardial capillary density, and M1/M2 macrophages. Western blotting was used to detect protein expression, and the proportion of monocytes was measured using flow cytometry.
Results
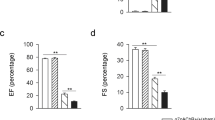
Activating the CAP with PNU282987 significantly improved cardiac function and reduced cardiac fibrosis and 28-day mortality after MI. On days 3 and 7 post-MI, PNU282987 reduced the percentage of peripheral CD172a + CD43low monocytes and the infiltration of M1 macrophages in the infarcted hearts, whereas it increased the recruitment of peripheral CD172a + CD43high monocytes and M2 macrophages. Conversely, MLA exerted the opposite effects. In vitro, PNU282987 inhibited M1 macrophage polarization and promoted M2 macrophage polarization in LPS + IFN-γ-stimulated RAW264.7 cells. These PNU282987-induced changes in LPS + IFN-γ-stimulated RAW264.7 cells were reversed by administering S3I-201.
Conclusion
Activating α7nAChR inhibits the early recruitment of pro-inflammatory monocytes/macrophages during MI and improves cardiac function and remodeling. Our findings suggest a promising therapeutic target for regulating monocyte/macrophage phenotypes and promoting healing after MI.








Similar content being viewed by others
References
Reyes-Retana JA, Duque-Ossa LC. Acute myocardial infarction biosensor: a review from bottom up. Curr Probl Cardiol. 2021;46(3): 100739.
Saleh M, Ambrose JA. Understanding myocardial infarction. F1000Res. 2018;7.
Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39(7):1073–84.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119(1):91–112.
Loi H, Kramar S, Laborde C, Marsal D, Pizzinat N, Cussac D, et al. Metformin Attenuates Postinfarction Myocardial Fibrosis and Inflammation in Mice. Int J Mol Sci. 2021;22 (17).
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59(2):153–63.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–47.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–6.
Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114(10):1611–22.
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96(8):881–9.
Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102(2):240–8.
Gombozhapova A, Rogovskaya Y, Shurupov V, Rebenkova M, Kzhyshkowska J, Popov SV, et al. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biomed Sci. 2017;24(1):13.
Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87.
Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107(8):1189–94.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96.
Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, et al. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension. 2011;57(2):298–307.
Wu SJ, Li YC, Shi ZW, Lin ZH, Rao ZH, Tai SC, et al. Alteration of cholinergic anti-inflammatory pathway in rat with ischemic cardiomyopathy-modified electrophysiological function of heart. J Am Heart Assoc. 2017;6 (9).
Li-Sha G, Xing-Xing C, Lian-Pin W, De-Pu Z, Xiao-Wei L, Jia-Feng L, et al. Right cervical vagotomy aggravates viral myocarditis in mice via the cholinergic anti-inflammatory pathway. Front Pharmacol. 2017;8:25.
Zhao M, He X, Bi XY, Yu XJ, Gil Wier W, Zang WJ. Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2013;108(3):345.
Fang J, Wang J, Chen F, Xu Y, Zhang H, Wang Y. alpha7nAChR deletion aggravates myocardial Infarction and enhances systemic inflammatory reaction via mTOR-signaling-related autophagy. Inflammation. 2019;42(4):1190–202.
Yang H, Liu S, Du H, Hong Z, Lv Y, Nie C, et al. Hydrogen attenuates myocardial injury in rats by regulating oxidative stress and nlrp3 inflammasome mediated pyroptosis. Int J Med Sci. 2021;18(14):3318–25.
Li HJ, Sun ZL, Pan YB, Xu MH, Feng DF. Effect of alpha7nAChR on learning and memory dysfunction in a rat model of diffuse axonal injury. Exp Cell Res. 2019;383(2): 111546.
Carlos DH, Bibiana Roselly CR, Angel UL, Laura MA, Kenya Karina SR, Jose Manuel CB, et al. Cognitive improvements in a rat model with polyunsaturated fatty acids EPA and DHA through alpha7-nicotinic acetylcholine receptors. Nutr Neurosci. 2022;25(4):791–800.
Deng Y, Guo SL, Wei B, Gao XC, Zhou YC, Li JQ. Activation of nicotinic acetylcholine alpha7 receptor attenuates progression of monocrotaline-induced pulmonary hypertension in rats by downregulating the NLRP3 inflammasome. Front Pharmacol. 2019;10:128.
Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Sillje HH, et al. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol. 2011;301(2):H459–68.
Obana M, Maeda M, Takeda K, Hayama A, Mohri T, Yamashita T, et al. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation. 2010;121(5):684–91.
Feuerborn R, Becker S, Poti F, Nagel P, Brodde M, Schmidt H, et al. High density lipoprotein (HDL)-associated sphingosine 1-phosphate (S1P) inhibits macrophage apoptosis by stimulating STAT3 activity and survivin expression. Atherosclerosis. 2017;257:29–37.
Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood). 2014;239(3):366–75.
Guo Q, Zhu X, Wei R, Zhao L, Zhang Z, Yin X, et al. miR-130b-3p regulates M1 macrophage polarization via targeting IRF1. J Cell Physiol. 2021;236(3):2008–22.
Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, et al. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104(11):1286–91.
Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay E, et al. Downregulation of microrna-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation. 2015;132(10):932–43.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310.
Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A. 2003;100(22):12929–34.
Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. Nf-kB transcription factor: role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. Clin Ter. 2001;152(4):249–53.
de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–51.
Joe Y, Kim HJ, Kim S, Chung J, Ko MS, Lee WH, et al. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2011;286(28):24735–42.
Espeland T, Lunde IG, B HA, Gullestad L, Aakhus S. Myocardial fibrosis. Tidsskr Nor Laegeforen. 2018;138 (16).
Czubryt MP, Hale TM. Cardiac fibrosis: Pathobiology and therapeutic targets. Cell Signal. 2021;85: 110066.
Zhang W, Wang L, Lu Z, Wang B, Li Y, Yang J, et al. Discovery of natural compounds for cardiac fibrosis by a transcriptome-based functional gene module reference approach. J Nat Prod. 2020;83(10):2923–30.
Zhao Y, Sun H, Li K, Shang L, Liang X, Yang H, et al. Cholinergic elicitation prevents ventricular remodeling via alleviations of myocardial mitochondrial injury linked to inflammation in ischemia-induced chronic heart failure rats. Mediators Inflamm. 2021;2021:4504431.
Li M, Zheng C, Kawada T, Inagaki M, Uemura K, Akiyama T, et al. Impact of peripheral alpha7-nicotinic acetylcholine receptors on cardioprotective effects of donepezil in chronic heart failure rats. Cardiovasc Drugs Ther. 2021;35(5):877–88.
Yang YH, Fang HL, Zhao M, Wei XL, Zhang N, Wang S, et al. Specific alpha7 nicotinic acetylcholine receptor agonist ameliorates isoproterenol-induced cardiac remodelling in mice through TGF-beta1/Smad3 pathway. Clin Exp Pharmacol Physiol. 2017;44(12):1192–200.
Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res. 2000;48(1):89–100.
Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6(1):5.
Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239(6):1573–84.
Liu Y, Niu XH, Yin X, Liu YJ, Han C, Yang J, et al. Elevated circulating fibrocytes is a marker of left atrial fibrosis and recurrence of persistent atrial fibrillation. J Am Heart Assoc. 2018;7 (6).
Rodness J, Mihic A, Miyagi Y, Wu J, Weisel RD, Li RK. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 2016;45:169–81.
Kakinuma Y, Furihata M, Akiyama T, Arikawa M, Handa T, Katare RG, et al. Donepezil, an acetylcholinesterase inhibitor against Alzheimer’s dementia, promotes angiogenesis in an ischemic hindlimb model. J Mol Cell Cardiol. 2010;48(4):680–93.
Han Z, Li L, Wang L, Degos V, Maze M, Su H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem. 2014;131(4):498–508.
Li XW, Wang H. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sci. 2006;78(16):1863–70.
Youssef ME, El-Mas MM, Abdelrazek HM, El-Azab MF. alpha7-nAChRs-mediated therapeutic angiogenesis accounts for the advantageous effect of low nicotine doses against myocardial infarction in rats. Eur J Pharmacol. 2021;898: 173996.
Yu JG, Song SW, Shu H, Fan SJ, Liu AJ, Liu C, et al. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine-alpha7-nicotinic ACh receptor in rats. Eur Heart J. 2013;34(30):2412–20.
Giannopoulou C, Geinoz A, Cimasoni G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J Clin Periodontol. 1999;26(1):49–55.
Sadigh-Eteghad S, Talebi M, Mahmoudi J, Babri S, Shanehbandi D. Selective activation of alpha7 nicotinic acetylcholine receptor by PHA-543613 improves Abeta25-35-mediated cognitive deficits in mice. Neuroscience. 2015;298:81–93.
Skinovsky J, Malafaia O, Chibata M, Tsumanuma F, Panegalli FF, Martins MV. The influence of nicotine in healing of small bowel anastomoses in rats: angiogenesis and miofibroblasts. Rev Col Bras Cir. 2016;43(2):87–92.
Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol. 1995;66(12):1056–64.
Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure—activity relationship. J Med Chem. 2006;49(14):4425–36.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65.
Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28.
ter Horst EN, Hakimzadeh N, van der Laan AM, Krijnen PA, Niessen HW, Piek JJ. Modulators of macrophage polarization influence healing of the infarcted myocardium. Int J Mol Sci. 2015;16(12):29583–91.
Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68(6):931–49.
Kiss A, Tratsiakovich Y, Mahdi A, Yang J, Gonon AT, Podesser BK, et al. Vagal nerve stimulation reduces infarct size via a mechanism involving the alpha-7 nicotinic acetylcholine receptor and downregulation of cardiac and vascular arginase. Acta Physiol (Oxf). 2017;221(3):174–81.
Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116(6):1101–12.
Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115(2):284–95.
Baez-Pagan CA, Delgado-Velez M, Lasalde-Dominicci JA. Activation of the macrophage alpha7 nicotinic acetylcholine receptor and control of inflammation. J Neuroimmune Pharmacol. 2015;10(3):468–76.
Wu J, Jiao ZY, Zhang Z, Tang ZH, Zhang HH, Lu HL, et al. Cross-talk between alpha7 nAChR-mediated cholinergic pathway and acylation stimulating protein signaling in 3T3-L1 adipocytes: role of NFkappaB and STAT3. Biochem Cell Biol. 2015;93(4):335–42.
Funding
This study was supported by the National Natural Science Foundation of China (No.82070333), the Zhejiang Provincial Natural Science Foundation of China (No.LY21H020011), and the Wenzhou Science and Technology Bureau Major Scientific Research Project (No.ZY2020018).
Author information
Authors and Affiliations
Contributions
JL and LL designed the research, wrote the paper; XN and RL conducted animal experiments; XL, RH and XL conducted cell experiments; SW and FL analyzed the data and contributed to revise the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Mauro Teixeira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, XH., Liu, RH., Lv, X. et al. Activating α7nAChR helps post-myocardial infarction healing by regulating macrophage polarization via the STAT3 signaling pathway. Inflamm. Res. 72, 879–892 (2023). https://doi.org/10.1007/s00011-023-01714-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01714-2