Abstract
Aim
To explore the role and mechanism of human adipose-derived mesenchymal stem cells (hAD-MSCs) in the treatment of osteoarthritis (OA).
Methods
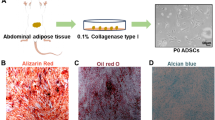
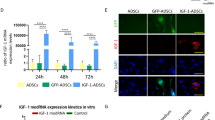
OA hulth model of Sprague Dawley (SD) rats and 20 ng/ml TNF-α treated chondrocytes were used as models of OA in vivo and in vitro, respectively. hAD-MSCs were administrated in the articular cavity by injection in vivo and co-cultured with chondrocytes using transwell in vitro. Haematoxylin and eosin staining and Safranin-O/Fast green staining were performed to detect tissue destruction and histopathology. Scanning electron microscopy and transmission electron microscopy were used to observe the ultrastructure of chondrocytes. The pyroptosis signaling pathway-related proteins were detected by immunohistochemistry, immunofluorescence, qRT-PCR and Western blot. And small interference technology was used to study the mechanism in depth.
Results
hAD-MSCs could delay the development of rat OA, improve the pathological changes of joints, inhibit the expression of NLRP3, Caspase1, GSDMD and TNFR1. In vitro, the expression of pyroptosis signal proteins in chondrocytes was significantly elevated when stimulated with TNF-α, the level of inflammatory factors such as IL-1β, IL-18 was increased, and the cell morphology was significantly destroyed. While co-cultured with hAD-MSCs, these syndromes were reversed. Knockout of TNFR1 also returned the upregulation of pyroptosis signals which caused by TNF-α.
Conclusion
These results demonstrated that hAD-MSCs could inhibit pyroptosis signaling pathway of chondrocytes induced by TNF-α, which have raised our understanding of the role of hAD-MSCs as promising therapy for the management of OA.








Similar content being viewed by others
References
O'neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep. 2018;16(5):611–616.
Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–8.
Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234: 116786.
Zhang W, Ouyang H, Dass CR, et al. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040.
Choi MC, Jo J, Park J, et al. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells, 2019,8(7).
Peng Z, Sun H, Bunpetch V, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268: 120555.
Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185–96.
Ye B, Chen X, Dai S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019;13:975–90.
Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75.
Zhang Y, Lin Z, Chen D, et al. CY-09 attenuates the progression of osteoarthritis via inhibiting NLRP3 inflammasome-mediated pyroptosis. Biochem Biophys Res Commun. 2021;553:119–25.
Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8.
Mcallister MJ, Chemaly M, Eakin AJ, et al. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarth Cartil. 2018;26(5):612–9.
Olee T, Hashimoto S, Quach J, et al. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162(2):1096–100.
Bougault C, Gosset M, Houard X, et al. Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models. Arthritis Rheum. 2012;64(12):3972–81.
Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–8.
Harrell CR, Markovic BS, Fellabaum C, et al. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.
Zhao X, Zhao Y, Sun X, et al. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8: 575057.
Dubey NK, Wei H-J, Yu S-H, et al. Adipose-derived stem cells attenuates diabetic osteoarthritis via inhibition of glycation-mediated inflammatory cascade. Aging Dis. 2019;10(3):483–96.
Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–18.
Taléns-Visconti R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12(36):5834–45.
Zhang HT, Liu ZL, Yao XQ, et al. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy. 2012;14(10):1203–14.
Gao J, Yuan J, Liu Q, et al. Adipose-derived stem cells therapy effectively attenuates PM(2.5)-induced lung injury. Stem Cell Res Ther. 2021;12(1):355.
Wang J, Ren H, Yuan X, et al. Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res. 2018;48(3):E194-e202.
Robert AW, Marcon BH, Dallagiovanna B, et al. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: a comparative transcriptome approach. Front Cell Dev Biol. 2020;8:561.
Liu F, Yang H, Li D, et al. Punicalagin attenuates osteoarthritis progression via regulating Foxo1/Prg4/HIF3α axis. Bone. 2021;152: 116070.
Gerwin N, Bendele AM, Glasson S, et al. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24-34.
Wu X, Ren G, Zhou R, et al. The role of Ca(2+) in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019;99(4):499–513.
Shi J, Liang J, Guo B, et al. Adipose-derived stem cells cocultured with chondrocytes promote the proliferation of chondrocytes. Stem Cells Int. 2017;2017:1709582.
Xia B, Di C, Zhang J, et al. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505.
Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87.
Li Z, Huang Z, Zhang H, et al. P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-κB/NLRP3 crosstalk. Oxid Med Cell Longev. 2021;2021:8868361.
Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020:8293921.
Wang C, Gao Y, Zhang Z, et al. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine. 2020;78: 153305.
Zhang L, Zhang L, Huang Z, et al. Increased HIF-1α in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis. Oxid Med Cell Longev. 2019;2019:6326517.
Zhang L, Xing R, Huang Z, et al. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediators Inflamm. 2019;2019:2165918.
Sharma D, Malik A, Guy C, et al. TNF/TNFR axis promotes pyrin inflammasome activation and distinctly modulates pyrin inflammasomopathy. J Clin Invest. 2019;129(1):150–62.
Mcgeough MD, Wree A, Inzaugarat ME, et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest. 2017;127(12):4488–97.
An S, Hu H, Li Y, et al. Pyroptosis plays a role in osteoarthritis. Aging Dis. 2020;11(5):1146–57.
Clavijo-Cornejo D, Martínez-Flores K, Silva-Luna K, et al. The overexpression of NALP3 inflammasome in knee osteoarthritis is associated with synovial membrane prolidase and NADPH oxidase 2. Oxid Med Cell Longev. 2016;2016:1472567.
Tseng WY, Huang YS, Lin HH, et al. TNFR signalling and its clinical implications. Cytokine. 2018;101:19–25.
De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80.
Childs PG, Reid S, Salmeron-Sanchez M, et al. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochem J. 2020;477(17):3349–66.
Lee WS, Kim HJ, Kim KI, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–11.
Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30.
Wang W, Cao W. Treatment of osteoarthritis with mesenchymal stem cells. Sci China Life Sci. 2014;57(6):586–95.
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340.
Liang H, Ding X, Yu Y, et al. Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp Cell Res. 2019;383(1): 111465.
Ke F, Zhang L, Liu Z, et al. Soluble tumor necrosis factor receptor 1 released by skin-derived mesenchymal stem cells is critical for inhibiting Th17 cell differentiation. Stem Cells Transl Med. 2016;5(3):301–13.
Acknowledgements
We are very grateful to Fanjiang Meng, who was employed by Bopin Biopharma Co., Ltd, for providing us with human adipose-derived mesenchymal stem cells. And we are particularly grateful to the support of the Center for Scientific Research of Anhui Medical University.
Author information
Authors and Affiliations
Contributions
LX: conceptualization, methodology, software, validation, writing—original draft. FZ: investigation, resources. Gang cheng: investigation, resources. XY: investigation, resources. YW: investigation, resources. HW: writing—review and editing, formal analysis. QW: validation, formal analysis. JC: data curation, visualization. JK: data curation, visualization. YC: data curation, visualization. WW: writing—review and editing, project administration, supervision. SY: conceptualization, methodology, writing—review and editing, project administration, funding acquisition, supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Zhang, F., Cheng, G. et al. Attenuation of experimental osteoarthritis with human adipose-derived mesenchymal stem cell therapy: inhibition of the pyroptosis in chondrocytes. Inflamm. Res. 72, 89–105 (2023). https://doi.org/10.1007/s00011-022-01655-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01655-2